Vaping Products Promotion Regulations: SOR/2020-143
Canada Gazette, Part II, Volume 154, Number 14
Registration
SOR/2020-143 June 26, 2020
TOBACCO AND VAPING PRODUCTS ACT
P.C. 2020-488 June 25, 2020
Her Excellency the Governor General in Council, on the recommendation of the Minister of Health, pursuant to section 33 footnote a of the Tobacco and Vaping Products Act footnote b, makes the annexed Vaping Products Promotion Regulations.
TABLE OF PROVISIONS
Vaping Products Promotion Regulations
Definition
1 Definition of Act
PART 1
Advertising and Point of Sale Promotion
Advertising
2 Advertising — young persons
Point of Sale Promotion
3 Display — vaping product
4 Display — package
5 Display — brand element
6 Visual advertising — general conditions
7 Signs — specific conditions
PART 2
Required Information in Advertising
Health Warning
8 Advertising — list of health warnings
9 Exceptions to section 8
10 Single health warning
11 Official languages
Attribution
12 Attribution of health warning
Presentation of Required Information
Visual Advertising
General Requirements
13 Application of sections 14 to 23
14 Definition of display area
15 Required information — placement
16 Required information — visibility and legibility
17 Requirement — rectangular border
18 Display area
19 Presentation of health warning
20 Official languages — placement
21 Health warning — legibility
22 Attribution
23 Attribution — legibility
Advertising by Means of Telecommunication
24 Non-application of sections 14 to 23
25 Required information — display
26 Required information — visibility and legibility
27 Official languages — display
28 Health warning — legibility
29 Attribution
30 Attribution — legibility
Video Advertising
31 Required information
32 Health warning — minimum duration
Audio Advertising
33 Application of sections 34 to 36
34 Required information — placement
35 Requirements
36 Attribution
Audio-Visual Advertising by Video
37 Required information — audio-visual advertising by video
Coming into Force
38 30th day after publication
SCHEDULE
Vaping Products Promotion Regulations
Definition
Definition of Act
1 In these Regulations, Act means the Tobacco and Vaping Products Act.
PART 1
Advertising and Point of Sale Promotion
Advertising
Advertising — young persons
2 (1) For the purposes of section 30.701 of the Act, a vaping product or a vaping product-related brand element must not be promoted by means of advertising done in a manner that allows the advertising to be seen or heard by young persons.
Exceptions
(2) Subsection (1) does not apply to the following types of advertising:
- (a) subject to paragraph (b), visual advertising that is located at the point of sale, if the conditions set out in paragraphs 6(1)(a) to (d) are met;
- (b) advertising on a sign that is located at a point of sale that is a retail establishment where vaping products are sold if
- (i) the conditions set out in paragraphs 7(1)(a) to (e) are met, or
- (ii) the sign indicates only the availability at the establishment and price of vaping products and provincial legislation that governs the retail establishment applies to signs promoting vaping products;
- (c) advertising in a publication that is addressed and sent to an adult who is identified by name; and
- (d) advertising in a publication that is provided on request to an adult at a point of sale that is a retail establishment where vaping products are sold.
Point of Sale Promotion
Display — vaping product
3 (1) For the purposes of section 30.8 of the Act, a vaping product must not be displayed, at the point of sale, in a manner that allows it to be seen by young persons.
Exception — provincial legislation
(2) Subsection (1) does not apply to a point of sale that is a retail establishment where vaping products are sold if provincial legislation that governs the retail establishment prohibits vaping products from being displayed in a manner that allows them to be seen by young persons.
Display — package
4 (1) For the purposes of section 30.8 of the Act, the package of a vaping product must not be displayed, at the point of sale, in a manner that allows the package to be seen by young persons.
Exception — provincial legislation
(2) Subsection (1) does not apply to a point of sale that is a retail establishment where vaping products are sold if provincial legislation that governs the retail establishment prohibits the packages of vaping products from being displayed in a manner that allows the packages to be seen by young persons.
Display — brand element
5 (1) For the purposes of section 30.8 of the Act, a thing that displays a vaping product-related brand element must not be displayed, at the point of sale, in a manner that allows the brand element to be seen by young persons.
Exception — provincial legislation
(2) Subsection (1) does not apply to a point of sale that is a retail establishment where vaping products are sold if provincial legislation that governs the retail establishment prohibits, directly or indirectly, vaping product-related brand elements from being displayed in a manner that allows them to be seen by young persons.
Visual advertising — general conditions
6 (1) For the purposes of section 30.8 of the Act, a vaping product or a vaping product-related brand element must not, subject to subsection (2) and section 7, be promoted by means of a visual advertisement at the point of sale unless the following conditions are met:
- (a) only one such advertisement is located at the point of sale;
- (b) the advertisement indicates only the availability at the point of sale and price of vaping products;
- (c) the advertisement does not include any visual, sound or other effects that are likely to draw attention to it; and
- (d) the advertisement uses only black characters on a white background.
Exception — visibility
(2) Subsection (1) does not apply in respect of visual advertising done in a manner that does not allow the advertising to be seen by young persons.
Signs — specific conditions
7 (1) For the purposes of section 30.8 of the Act, a vaping product or a vaping product-related brand element must not, subject to subsections (2) and (3), be promoted by means of advertising on a sign that is located at a point of sale that is a retail establishment where vaping products are sold, unless the following conditions are met:
- (a) the sign indicates only the availability at the establishment and price of vaping products;
- (b) only one such sign is located at the establishment;
- (c) the sign does not include any visual, sound or other effects that are likely to draw attention to it;
- (d) the sign uses only black characters on a white background; and
- (e) the sign is rectangular and does not exceed 3 600 cm2 in area.
Exception — provincial legislation
(2) Subsection (1) does not apply if provincial legislation that governs the retail establishment applies to signs promoting vaping products.
Exception — visibility
(3) Subsection (1) does not apply in respect of advertising on a sign and that is done in a manner that does not allow the advertising to be seen by young persons.
PART 2
Required Information in Advertising
Health Warning
Advertising — list of health warnings
8 (1) For the purposes of section 30.7 of the Act, a vaping product or a vaping product-related brand element must not be promoted by means of advertising unless it conveys one of the health warnings set out in the document entitled List of Health Warnings for Vaping Product Advertising, as amended from time to time and published by the Government of Canada on its website.
Amended list
(2) If the List of Health Warnings for Vaping Product Advertising is amended, advertising may convey a health warning that was set out in the previous version of the List for a period of 60 days after the day on which the new version of that List is published by the Government of Canada.
Exceptions to section 8
9 Section 8 does not apply to the following types of advertising:
- (a) the advertising of a vaping product, if the vaping product is the subject of an authorization, including a licence, issued under the Food and Drugs Act authorizing its sale;
- (b) the advertising of a vaping product or a vaping product-related brand element, if provincial legislation that governs the advertising of such a product or brand element requires a health warning to be conveyed in the advertising;
- (c) advertising at the point of sale that indicates only the availability at the point of sale and price of vaping products; and
- (d) advertising on a sign that is located at a point of sale that is a retail establishment where vaping products are sold and that indicates the availability at the establishment and price of vaping products, as well as other information that is required by the provincial legislation referred to in subsection 7(2).
Single health warning
10 Every advertisement of a vaping product or a vaping product-related brand element must convey a single health warning.
Official languages
11 (1) If a health warning is conveyed in advertising that uses both official languages, or another language, it must be conveyed in both official languages.
Only one official language
(2) If a health warning is conveyed in advertising that uses only one official language, or one official language and another language, it must be conveyed in only that official language.
Attribution
Attribution of health warning
12 Every advertisement of a vaping product or a vaping product-related brand element must attribute the health warning to its source in accordance with section 22, 29 or 36.
Presentation of Required Information
Visual Advertising
General Requirements
Application of sections 14 to 23
13 Subject to section 24, sections 14 to 23 apply to all forms of visual advertising.
Definition of display area
14 For the purposes of section 15, subsection 17(1) and sections 18 and 19, display area, in respect of visual advertising, means the portion of the surface area of an advertisement on which the information required under this Part may be displayed and that,
- (a) in the case of visual advertising by video, occupies 100% of the surface area of the advertisement; and
- (b) in every other case, occupies — from the edge that is in the horizontal plane, that forms the upper limit of the advertisement and that extends from the left edge to the right edge of the advertisement — at least 20% of the surface area of the advertisement that is visible at first sight to consumers.
Required information — placement
15 Required information that is conveyed in visual advertising must be displayed on the display area.
Required information — visibility and legibility
16 Required information that is conveyed in visual advertising
- (a) must be clear and legible; and
- (b) must not be concealed or obscured.
Requirement — rectangular border
17 (1) Required information that is conveyed in visual advertising must be enclosed within a rectangular border that must be displayed on the display area in such a manner that it demarcates the information from any other information displayed on the advertisement.
Appearance — rectangular border
(2) The border must be the same colour as the type of the health warning and form a continuous line that has a uniform width of 3% of the length of the shortest side of the rectangle.
Display area
18 (1) Only the required information and the rectangular border that is referred to in section 17 may be displayed on the display area.
Background
(2) The display area must have a black or white background.
Presentation of health warning
19 The health warning must be centred in the display area, oriented parallel to the upper limit of the visual advertisement, and must occupy not less than 60% and not more than 70% of the display area.
Official languages — placement
20 If a health warning is conveyed in both official languages, each language version must be displayed immediately beside, below or above the other version, and the two texts must not be combined.
Health warning — legibility
21 (1) The health warning that is conveyed in visual advertising must be displayed in a standard sans serif type that
- (a) is not compressed, expanded or decorative;
- (b) as illustrated in the schedule, has a large x-height relative to the ascender or descender of the type; and
- (c) is black, on a background that is white, or white, on a background that is black.
Characters in text — health warning
(2) Each character in the text must have the same font and type size.
Text of health warning
(3) The health warning must be displayed in such a manner that
- (a) the first word is in upper case letters and bold type;
- (b) the remaining text is capitalized in the same manner as in the List of Health Warnings for Vaping Product Advertising and it is not in bold type; and
- (c) if the health warning is displayed on more than one line of text, the letters in each word appear on the same line of text.
Attribution
22 The attribution “Health Canada” must be displayed immediately beside or below the English version of a health warning that is displayed in visual advertising, and the attribution “Santé Canada” must be displayed immediately beside or below the French version of the health warning.
Attribution — legibility
23 (1) The attribution of a health warning must be displayed in such a manner that
- (a) it meets the requirements set out in subsections 21(1) and (2);
- (b) it is not in bold type; and
- (c) the height of the type of the attribution is equal to the x-height, as illustrated in the schedule, of a lower case letter displayed in the text of the health warning.
Measurement of height of type
(2) The height of the type must be determined by measuring an upper case letter or a lower case letter that has an ascender or a descender, such as “b” or “p”.
Characters in text
(3) Each character in the text of the attribution must have the same font as the text of the health warning.
Advertising by Means of Telecommunication
Non-application of sections 14 to 23
24 (1) Sections 14 to 23 do not apply to required information that is conveyed in visual advertising transmitted by a means of telecommunication that does not allow the display of required information in accordance with the requirements set out in those sections.
Application of sections 25 to 30
(2) Sections 25 to 30 apply to required information that is conveyed in visual advertising transmitted by a means of telecommunication that does not allow the display of required information in accordance with the requirements set out in sections 14 to 23.
Required information — display
25 Required information must be displayed at the beginning of the advertising.
Required information — visibility and legibility
26 Required information
- (a) must be clear and legible; and
- (b) must not be concealed or obscured.
Official languages — display
27 If a health warning is conveyed in both official languages, each language version must be displayed before or after the other version.
Health warning — legibility
28 (1) The health warning must be displayed in a standard sans serif type that is not compressed, expanded or decorative.
Characters in text
(2) Each character in the text of the health warning must have the same font and type size.
Text of health warning
(3) The health warning must be displayed in such a manner that
- (a) the text is presented in a consolidated manner, without any intervening words or images;
- (b) the first word is in upper case letters;
- (c) the remaining text is capitalized in the same manner as in the List of Health Warnings for Vaping Product Advertising; and
- (d) if the health warning is displayed on more than one line of text, the letters in each word appear on the same line of text.
Attribution
29 The attribution “Health Canada” must be displayed immediately after the English version of the health warning that is displayed in the advertising, and the attribution “Santé Canada” must be displayed immediately after the French version of the health warning.
Attribution — legibility
30 The attribution of a health warning must be displayed
- (a) in a standard sans serif type that is not compressed, expanded or decorative; and
- (b) in the same font and type size as the health warning.
Video Advertising
Required information
31 Required information that is conveyed in visual advertising by video must be displayed at the end of the advertising.
Health warning — minimum duration
32 A health warning that is conveyed in visual advertising by video must be displayed for at least
- (a) four seconds, if the health warning is displayed in only one official language; or
- (b) eight seconds, if the health warning is displayed in both official languages.
Audio Advertising
Application of sections 34 to 36
33 Sections 34 to 36 apply to all kinds of audio advertising.
Required information — placement
34 Required information that is conveyed in audio advertising must be conveyed at the end of the audio advertising and must not be combined with any other audio information.
Requirements
35 The following requirements apply to the health warning that is conveyed in audio advertising:
- (a) the health warning must be conveyed in its entirety at the same speed, volume and tone as the main message, without any word being emphasized more than any other;
- (b) the health warning must be conveyed at the same speed, volume and tone in both official languages, if the advertising uses both official languages or another language; and
- (c) the health warning must be conveyed without any music or background sound.
Attribution
36 The attribution “This is a Health Canada warning:” must immediately precede the English version of the health warning that is conveyed in audio advertising and the attribution “Ce message est une mise en garde de Santé Canada :” must immediately precede the French version of the health warning.
Audio-Visual Advertising by Video
Required information — audio-visual advertising by video
37 In the case of audio-visual advertising by video, the audio and visual components of required information must be conveyed simultaneously.
Coming into Force
30th day after publication
38 (1) Subject to subsection (2), these Regulations come into force on the 30th day after the day on which they are published in the Canada Gazette, Part II.
60th day after publication
(2) Sections 3 and 4 come into force on the 60th day after the day on which these Regulations are published in the Canada Gazette, Part II.
SCHEDULE
(Paragraphs 21(1)(b) and 23(1)(c))
ILLUSTRATION — STANDARD SANS SERIF TYPE

Image description
The height of the lower case letter x is the x-height. The part of the lower case letter b that is above the x-height is called an ascender. The part of the lower case letter p that is below the x-height is called a descender.
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Executive summary
Issues: A rapid increase in youth vaping has been observed in Canada. Young persons are being exposed to vaping product-related harms, including those related to nicotine exposure which can result in a dependence on nicotine and an increased risk of tobacco use. Health Canada has identified vaping product-related promotional activities as being one of the key factors that have contributed to the rise in youth vaping.
Description: The Vaping Products Promotion Regulations (the Regulations) set out measures to reduce the impact of vaping product promotion on young persons and non-users of tobacco products. The Regulations (1) prohibit the promotion of vaping products and vaping product-related brand elements by means of advertising that is done in a manner that can be seen or heard by young persons, including the display of vaping products at points of sale in a manner that allows them to be seen by young persons; and (2) require that all vaping product advertising convey a health warning about the health hazards of vaping product use.
Cost-benefit statement: The Regulations will result in total incremental costs estimated at $7.7 million present value (PV) over 10 years (or $1.1 million annually), consisting of $4.2 million (or $0.6 million annually) in industry costs and $3.5 million (or $0.5 million annually) in government costs. The monetized costs to industry are associated with complying with the prohibition on the display of vaping products at points of sale in a manner that allows them to be seen by youth and the requirement that a health warning be conveyed in those advertisements not prohibited under the Regulations. The government costs are associated with the costs to monitor compliance and conduct enforcement activities under the Regulations. The benefits of the Regulations include: protecting youth from inducements to use vaping products, which could lead to nicotine exposure and an increased risk of tobacco use; enhancing public awareness of the health hazards of using vaping products; and, supporting Canada’s Tobacco Strategy that aims to reduce the burden of disease and death of tobacco use including its impact on the public health system. Even if they cannot be directly quantified, the benefits are expected to outweigh the costs of the Regulations.
One-for-one rule and small business lens: The small business lens applies. There is no administrative burden on businesses as a result of the Regulations. Therefore, the one-for-one rule does not apply.
Domestic and international coordination and cooperation: The Regulations apply prohibitions and requirements on the promotion of vaping products that do not conflict with provincial and territorial promotion restrictions, including in the retail environment. With regard to the requirement that all vaping product advertisements convey a health warning, the Regulations will not apply where a vaping product advertisement must convey a health warning required by a provincial or territorial legislation, as is the case in Quebec.
Due to the uniqueness of the legislative framework set out in the Tobacco and Vaping Products Act (TVPA), the Regulations will not align with measures in the United States that are less restrictive on vaping promotions and that require a different health warning in advertisements of vaping products.
Issues
A rapid increase in youth vaping has been observed in Canada. Data from the 2018–2019 Canadian Student Tobacco, Alcohol and Drugs Survey (CSTADS) indicates that the prevalence of vaping has doubled among students compared to the previous survey in 2016–2017. Young persons are being exposed to vaping product-related harms, including those related to nicotine exposure, which can result in a dependence on nicotine and an increased risk of tobacco use.
Since the enactment of the Tobacco and Vaping Products Act (TVPA), vaping advertising has been observed on television, on social media and other digital platforms, at events, on outdoor signs and at points of sale.
Evidence suggests that Canadians know very little about the harms of using vaping products. Health Canada has also observed that not all vaping product advertisements display a health warning, and where such a warning is displayed, it is not prominently displayed and its content is not consistent across all advertisements.
Background
Vaping — Survey data
Data from the 2018–2019 CSTADS indicates that the prevalence (past 30 days) of vaping had doubled among students compared to the previous survey in 2016–2017. footnote 1 Twenty percent of students (418,000) in grades 7 to 12 (secondary I through V in Quebec) had used an e-cigarette footnote 2 in the past 30 days, double the 10% from 2016–2017. In 2018–2019, the past-30-day prevalence was 11% (115,000) among students in grades 7 to 9 (secondary I to III in Quebec) and 29% (304,000) among students in grades 10 to 12 (secondary IV and V in Quebec). Further data is presented in Figure 1. It was found that frequency of use is high, particularly in the upper grades: the prevalence of daily or almost daily e-cigarette use was 13% (133,000) among students in grades 10 to 12. As a comparison, the prevalence of daily or almost daily cigarette use among students in grades 10 to 12 was 1% (14,000) in 2018–2019 (Figure 2).
Figure 1: Past-30-day e-cigarette use grouped by grade (CSTADS)
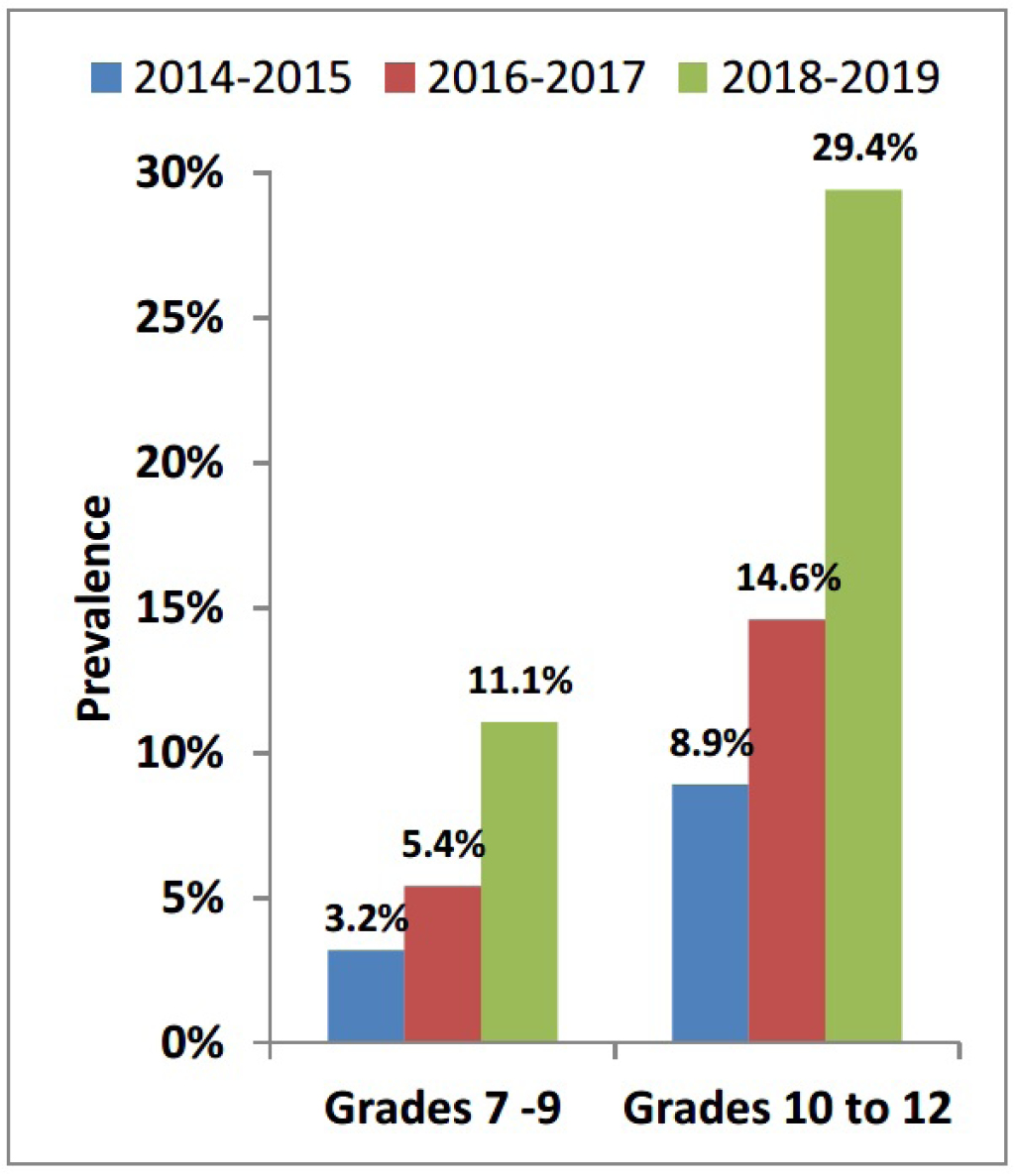
Image description
| Year | Prevalence of past-30-day use of e-cigarettes by students in grades 7 to 9 (CSTADS) |
|---|---|
| 2014-2015 | 3.2 % |
| 2016-2017 | 5.4 % |
| 2018-2019 | 11.1 % |
| Year | Prevalence of past-30-day use of e-cigarettes by students in grades 10 to 12 (CSTADS) |
|---|---|
| 2014-2015 | 8.9 % |
| 2016-2017 | 14.6 % |
| 2018-2019 | 29.4 % |
Figure 2: Daily cigarette smoking and daily/almost daily e-cigarette use, grades 10–12 (2018–2019 CSTADS)
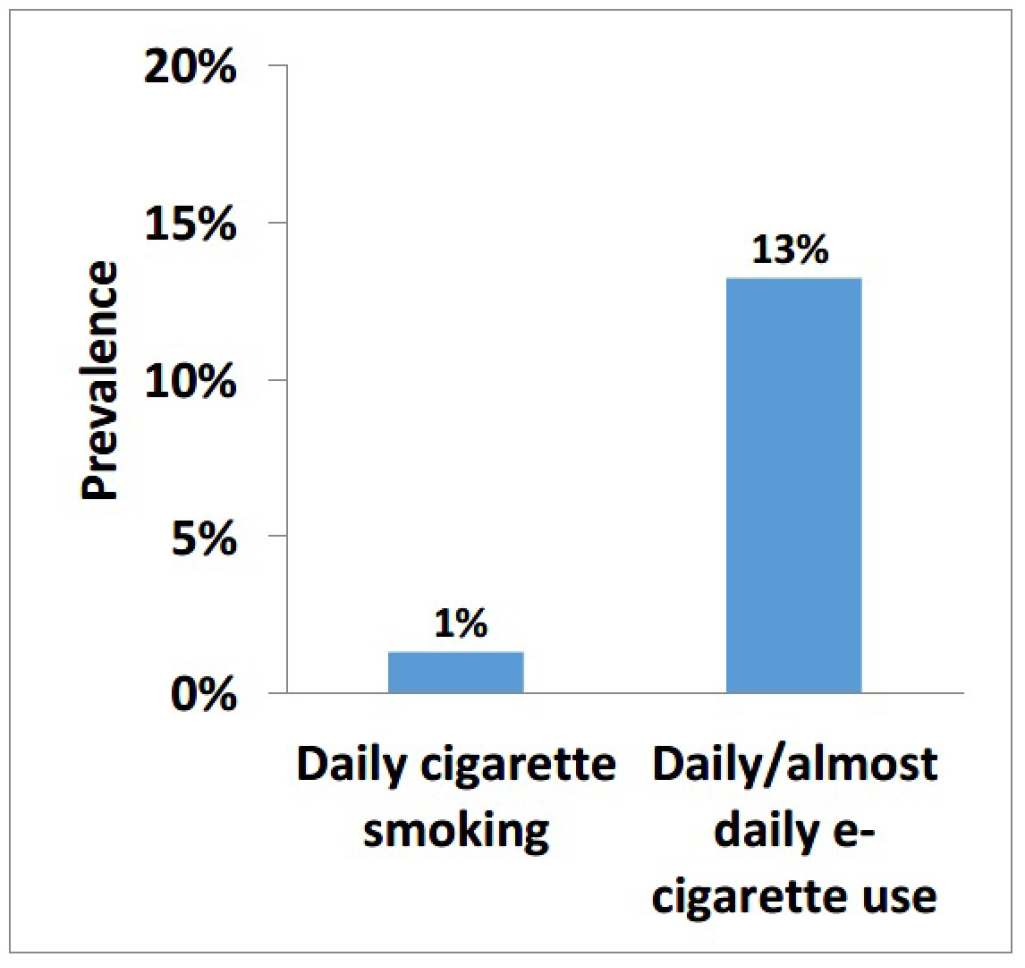
Image description
| By students in grades 10 to 12 (2018–2019 CSTADS) | Prevalence |
|---|---|
| Daily cigarette smoking | 1 % |
| Daily/almost daily e-cigarette use | 13 % |
Health concerns and nicotine addiction
Vaping products are harmful. They emit an aerosol that contains potentially harmful chemicals. The inhalation of these emissions into the lungs may have a negative impact on the health, particularly for youth. While they present risks, vaping products offer a less harmful alternative for persons who smoke if they switch completely to vaping.
Most vaping products contain nicotine. Children and youth are especially susceptible to the harmful effects of nicotine, including addiction. Youth can become dependent on nicotine at lower levels of exposure than adults do. Exposure to nicotine during adolescence can also negatively alter brain development, including long-term effects on memory and concentration abilities.
Vaping-associated lung illness
The Regulations do not address the recent emergence of vaping-associated lung illness that has been observed in Canada and in the United States in 2019 and 2020. footnote 3, footnote 4 Emergency department visits related to e-cigarettes continue to decline, after sharply increasing in August 2019 and peaking in September 2019. The U.S. Centers for Disease Control and Prevention has concluded that vitamin E acetate, an additive in some tetrahydrocannabinol (THC)-containing vaping products, is the primary cause of the outbreak. The evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC or non-THC products, in some of the reported cases.
As a precautionary measure, Health Canada and the Public Health Agency of Canada recommend that those who are concerned about the health risks related to vaping should consider not vaping. For persons who vape, it is recommended that they should not use vaping products that have been obtained from illegal or unregulated sources, including illegal cannabis products, as they are not subject to any controls or oversight and may pose additional risks to health and safety. The Government of Canada will take action, as appropriate, to protect the health and safety of Canadians.
Vaping — A new legislative framework
In response to the 2015 report of the House of Commons’ Standing Committee on Health entitled Vaping: Toward a Regulatory Framework for E-Cigarettes, a new legislative framework was established by Parliament. An Act to amend the Tobacco Act and the Non-smokers’ Health Act and to make consequential amendments to other Acts received royal assent on May 23, 2018. As a consequence, vaping products are regulated under the TVPA and either the Food and Drugs Act or the Canada Consumer Product Safety Act, depending on whether or not the product is marketed for therapeutic use. The provisions of the TVPA apply to all vaping products, including those regulated under the Food and Drugs Act, except where they are expressly excluded from the application of the TVPA and some of its provisions (e.g. through the Regulations Excluding Certain Vaping Products Regulated under the Food and Drugs Act from the Application of the Tobacco and Vaping Products Act).
Objectives and scope of the Tobacco and Vaping Products Act
The overall objective of the TVPA with respect to vaping products is to prevent vaping product use from leading to the use of tobacco products by young persons and non-users of tobacco products. Specifically, it aims to (1) protect young persons and non-users of tobacco products from inducements to use vaping products; (2) protect the health of young persons and non-users of tobacco products from exposure to and dependence on nicotine that could result from the use of vaping products; (3) protect the health of young persons by restricting access to vaping products; (4) prevent the public from being deceived or misled with respect to the health hazards of using vaping products; and (5) enhance public awareness of those hazards.
To this end, the TVPA regulates, in addition to tobacco, the manufacture, sale, labelling and promotion of vaping products. Several provincial and territorial jurisdictions have also adopted measures to regulate vaping products, to varying degrees and through different approaches. These generally include measures to prohibit vaping product sales to youth and restrict promotional activities at retail establishments.
The TVPA prohibits vaping product advertising that could be appealing to young persons and lifestyle advertising. The TVPA also prohibits certain types of vaping product promotion, such as sponsorship promotion, testimonials or endorsements as well as the promotion of flavour descriptors that are appealing to youth. Vaping advertising can be further restricted by regulations.
Current research on vaping
The report entitled Public Health Consequences of E-Cigarettes, published in 2018 by the U.S. National Academies of Sciences, Engineering, and Medicine (NASEM), footnote 5 represents expert consensus resulting from an independent, systematic review of a high volume of peer-reviewed scientific studies. The report offers three conclusions that are of particular significance in supporting the need to further protect youth and non-tobacco users: (1) there is substantial evidence that the use of an e-cigarette results in symptoms of dependence; (2) there is conclusive evidence that in addition to nicotine, most e-cigarette products contain and emit numerous potentially toxic substances; and (3) there is substantial evidence that e-cigarette use increases the risk of ever using combustible tobacco cigarettes among youth and young adults.
Canada’s Tobacco Strategy
Tobacco use is the leading preventable cause of disease and premature death in Canada. It is a known or probable cause of more than 40 debilitating and often fatal diseases of the lungs, heart, and other organs, and is responsible for approximately 45 000 premature deaths every year in Canada. Tobacco products contain nicotine, a highly addictive substance that is responsible for tobacco dependence and consequent repeated long-term use that results in chronic exposure to harmful chemicals. Health and economic costs associated with tobacco use in Canada are estimated at $16.2 billion annually (based on 2012 data). footnote 6
Canada’s Tobacco Strategy (CTS), introduced in 2018, features broad, population-based approaches to achieve the ambitious target of less than 5% tobacco use prevalence by 2035, with targeted approaches focused on specific populations suffering from high levels of tobacco use. One of the Strategy’s objectives is to protect youth and non-tobacco users from nicotine addiction.
Factors contributing to the rise in youth vaping
There are a number of factors that have contributed to a rapid and significant increase in youth uptake of vaping products since the enactment of the TVPA: an increase in promotional activities relating to vaping products including on social media, the introduction of high-nicotine-concentration products in the market, the use of a wide variety of flavours and design features that make vaping products appealing to youth.
Canada’s public health achievements in tobacco control are at risk of being eroded if young persons who experiment with vaping products develop a dependence on nicotine, particularly those who would not otherwise have tried smoking.
Youth exposure to advertising
An increase in vaping product advertising has been observed on television, on social media and other digital platforms, at events, on outdoor signs and at points of sale. Studies indicate that youth and young adults are particularly vulnerable to the effects of commercial promotions. Youth who are exposed to vaping product advertisements exhibit a greater openness to try and a greater likelihood of using vaping products. While the TVPA prohibits certain types of vaping promotion, vaping product advertising is still permitted in most public places. These promotions are believed to have contributed to the inducements that lead young persons to experiment with vaping products.
Low awareness of harms
Evidence suggests that Canadians know very little about the harms of vaping. The 2017 Canadian Tobacco, Alcohol and Drugs Survey indicated that almost one in four Canadians were unaware of the harms of vaping by using an e-cigarette once in a while (23%) or on a regular basis (24%). Health Canada has observed that not all vaping product advertisements display a health warning, and where such a warning is displayed, it is not prominently displayed and its content is not consistent across all advertisements.
Addressing the rise in youth vaping
Health Canada is focusing its efforts under the CTS to address the rise of youth vaping. To that end, the department is considering a suite of regulatory measures under the TVPA to address youth vaping. Health Canada is also conducting a targeted youth-oriented public information campaign with national reach to increase youth awareness of the harms of vaping. In addition, grants and contributions funding of $14 million have been allocated over four years to address tobacco use and youth vaping through the Substance Use and Addictions Program.
Objective
The objective of Part 1 of the Regulations is to protect young persons from inducements to use vaping products by prohibiting advertising that can be seen or heard by them, including the display of vaping products and vaping product-related brand elements at points of sale.
The objective of Part 2 of the Regulations is to enhance public awareness about the health hazards or health effects of using vaping products by requiring that advertising convey a health warning that would enable adults to make an informed choice regarding the use of these products.
Description
The Regulations are presented in two parts.
Part 1 — Advertising and Point of Sale Promotion
Advertising
This part prohibits the promotion of a vaping product or a vaping product-related brand element by means of advertising done in a manner that allows the advertising to be seen or heard by young persons. Hence, advertising in places such as recreational facilities, public transit facilities, broadcast media, in publications or online will be prohibited, if it is done in a manner that allows it to be seen or heard by young persons. This would also apply to the display of vaping product-related brand elements on retail storefronts signs and banners (section 2 of the TVPA defines “brand element” as including a term or logo that is reasonably associated with a product or brand – including a brand name or trade-name and defines a “young person” as a person under eighteen years of age).
Advertising that is communicated in a way that ensures that it cannot be seen or heard by a young person remains permitted anywhere, as long as it is compliant with all other applicable provisions of the TVPA and the Regulations. Therefore, subject to limited exceptions set out in the Regulations, vaping product advertising will only be permitted if it is displayed or accessed in a manner that ensures that it cannot be seen or heard by young persons. This means that prior to providing access to vaping advertising online, reasonable steps must be taken by the regulated parties to ensure that the age of the visitor is diligently verified, which will depend on the technological means and services available to effectively verify the age of individuals who are seeking to view their ads online. Hence, simply requiring visitors to “check the box” to attest of their age or to self-declare about their date of birth or age on a website or social media page before they can access the advertising would not be considered sufficient to prevent youth access to a vaping promotion.
These restrictions will also apply to advertising of vaping products regulated by the Food and Drugs Act that are not excluded from the application of the TVPA (e.g. those not excluded through the Regulations Excluding Certain Vaping Products Regulated Under the Food and Drugs Act from the Application of the Tobacco and Vaping Products Act).
The exceptions for advertising on signs at points of sale and in publications are as follows:
Signs at points of sale
For the purpose of the Regulations, points of sale include retail establishments and online stores. Retail establishments include all physical locations where vaping products are sold, including gas and convenience (G&C) stores, vape shops, temporary points of sale such as kiosks and stalls and any other physical locations where vaping products are sold to consumers.
Visual advertising that only indicates the availability and price of vaping products will be permitted at points of sale as long as it complies with the conditions set out in the Regulations with respect to the content, placement and form. These conditions will help ensure that this form of visual advertising would have a limited promotional impact on young persons.
The conditions are that there can only be one visual advertisement of this type per point of sale that is accessible to young persons. Only black characters on a white background are permitted and no visual, sound or other effects that may draw attention to it are permitted. For signs at retail establishments where vaping products are sold, additional requirements apply, e.g. the sign will have to be rectangular and not exceed 3 600 cm2 in area.
The placement, form and content conditions relating to signs at retail establishments do not apply where provincial or territorial legislation applies to the retail establishment and governs signs promoting vaping products. In such cases, the provincial or territorial requirements for signs on the price and availability of vaping products at retail establishments would apply.
Advertising in publications
Vaping product advertising in publications that are addressed and sent to a named adult are allowed. Advertising in publications, such as product brochures or pamphlets, are also permitted if the publication is provided on request to an adult at a retail establishment where vaping products are sold. These exceptions take into account the fact that the manner of communicating the advertising limits the likelihood that a young person would be exposed to it. Publications include print publications and electronic publications that are sent or accessed by means of telecommunication such as websites, applications, social media, text messages or other digital platforms. Advertising in these publications remains subject to all other applicable provisions set out in the TVPA.
Point of sale promotion
The display of vaping products and their packaging at points of sale, including those displayed at an online point of sale, are prohibited if they are displayed in a manner that would allow them to be seen by young persons. The Regulations also prohibit the display of a thing that shows a vaping product-related brand element at a point of sale in a manner that allows the brand element to be seen by young persons. For example, a baseball cap bearing a vaping product-related brand element can only be displayed at a point of sale if that brand element cannot be seen by a young person. Conversely, there is no prohibition on the display of vaping products, their packaging, or vaping product-related brand elements at points of sale that do not allow youth access. For online points of sale, reasonable steps must be taken by regulated parties to ensure that the age of the visitor is diligently verified prior to being able to see vaping products and their packaging.
For points of sales that are retail establishments and that allow youth access, the prohibition on the display of vaping products and their brand elements does not apply if provincial or territorial legislation applies to the establishment and prevents vaping products, their packaging or vaping product-related brand elements from being displayed in a manner that allows them to be seen by young persons.
Coming into force
The measures set out in Part 1 come into force 30 days after publication of the Regulations in the Canada Gazette, Part II, except the restrictions on the display of vaping products and their packaging at points of sale which come into force 60 days after publication of the Regulations.
Part 2 — Required Information in Advertising (Health Warning)
Part 2 of the Regulations requires that a health warning be conveyed in the advertising of a vaping product or vaping product-related brand element. It also sets out the conditions for the presentation of the health warning and of the attribution to Health Canada for both audio and visual vaping advertisements.
At the point of sale, a health warning does not have to be displayed in advertising that indicates only the availability and price of vaping products. Furthermore, the Regulations do not require the display of a health warning in vaping advertisements subject to provincial or territorial legislation that also requires the display of a prescribed health warning.
In the case of advertising for a vaping product authorized for sale under the Food and Drugs Act, an exception is provided in order not to conflict with the advertising conditions set out in that Act and associated regulations that apply to these products.
List of health warnings
The health warnings are listed in a document entitled List of Health Warnings for Vaping Product Advertising (the List) that is published by Health Canada on the Government of Canada website. The List is incorporated by reference in the Regulations and can be amended from time to time. This approach allows Health Canada to be more responsive to new scientific research on the health effects or health hazards of using vaping products. The document can be updated with additional health warnings, the removal of existing ones or amendments to their text. Health Canada can also modify the List to maintain their effectiveness. Regulated parties and interested stakeholders will be notified of any proposed changes to the document. The Regulations allow for a 60-day transition period following any change to the document to allow for the phasing out of advertisements conveying modified or deleted health warnings.
Official language requirements
Conditions relating to the presentation of the required information in advertising, such as size, format, placement, and use of official languages are prescribed in the Regulations. More specifically, every vaping product-related advertisement is required to convey a health warning. If the advertisement is only in one official language or in one official language and another language, the health warning must be conveyed only in the one official language. For advertisements that are in both official languages, or in a language other than an official language, the health warning must be conveyed in both official languages. The Regulations also set out a requirement to attribute the health warning to its source, i.e. to Health Canada.
General requirements and placement
The Regulations prescribe the manner of presenting health warnings in visual, audio and audiovisual advertisements. In all visual advertising, including by means of telecommunications, the display area for the health warning must occupy at least 20% of the surface area of the advertisement, the health warning and the attribution must be enclosed by a rectangular border in the display area, and there are requirements regarding the format and appearance of the health warning and its attribution.
The display area for the health warning in visual advertising by video must occupy 100% of the surface area of the advertisement, and the health warning itself must be conveyed at the end of the video. The health warning must remain visible for at least eight seconds, if displayed in both official languages and four seconds if displayed in one official language.
In the case of audio advertising, the health warning must be conveyed at the end of the advertisement and be communicated at the same speed, volume and tone as the rest of the audio advertising. In addition, the attribution “This is a Health Canada warning:” must precede the health warning that is conveyed in an audio advertising.
For audiovisual advertising by video, the requirements for both visual and audio advertising must be conveyed simultaneously.
Exception to the general requirements (visual advertising)
An exception to the requirements for the display of a health warning in a visual advertisement, as described above, is provided for certain visual advertising by means of telecommunication that does not allow the display of the required information in accordance with the requirements described above, including for example the requirement that it be enclosed within a rectangular border. Examples of such visual advertising may include advertising by means of text messaging applications that are limited to text and by email that are limited to plain text formats. In such cases only, a text-only health warning must be placed at the beginning of the advertisement.
Coming into force
The measures in Part 2 with regard to conveying health warning in advertisements come into force 30 days after publication of the Regulations in the Canada Gazette, Part II.
Regulatory development
Consultation
Consultation on advertising restrictions prior to prepublication in the Canada Gazette, Part I
In 2017, Health Canada published a consultation document setting out 10 proposals to regulate vaping products in Canada (2017 Consultation). The document was open for comments from the public and interested stakeholders for a 60-day period. A summary of the feedback received can be found in the Consultation Summary: Proposals for the Regulation of Vaping Products published by Health Canada in April 2018. footnote 7
Proposal 10 in the 2017 Consultation proposed to “establish regulations to help limit youth exposure to information and brand-preference advertising of vaping products. […] Restrictions would […] seek to limit advertising in or near locations that are attended predominantly by youth, such as schools, parks, recreational and sporting facilities. Restrictions would also be placed on advertising in certain media for example by either prohibiting advertisements on television and radio or restricting the times of the day when such advertisements may appear or be heard to limit youth exposure to them.”
In general, public health groups and non-governmental organizations (NGOs) were strong advocates for additional advertising restrictions, with many suggesting that vaping product advertising restrictions should be stricter than those being proposed and should align with those for tobacco products. Other suggestions included banning all promotion on television, radio, social media and billboards, at points of sale, and via the use of promotional emails and giveaways. They also advocated for a ban on all lifestyle advertising, including in age-restricted areas.
Those in the vaping industry (manufacturers and retailers) also expressed support for advertising restrictions in order to protect youth, while still allowing marketing of a reduced risk product to adult smokers. Some advocated that social media be allowed as an advertising medium, noting that they already have age-restricted access to their social media feeds to adults over the age of 19. Others suggested bans on vaping product advertising on television and radio at certain times of the day. One suggested banning all promotional materials near schools.
In February 2019, Health Canada published a consultation document entitled Notice of Intent – Potential Measures to Reduce the Impact of Vaping Products Advertising on Youth and Non-users of Tobacco Products (2019 Consultation) to seek feedback on selected regulatory measures under consideration, including measures to restrict the placement of advertisements where they can be seen or heard by youth as well as measures to prohibit the display of vaping products at retail establishments accessible to youth. The Notice of Intent was open for comments for a 45-day period. A summary of the feedback received can be found in the Consultation Summary: Notice of Intent – Potential Measures to Reduce the Impact of Vaping Products Advertising on Youth and Non-users of Tobacco Products published by Health Canada in July 2019. footnote 8
The majority of the respondents supported restrictions on the promotion of vaping products. NGOs, associations of health professionals, local and regional health authorities, municipalities and the general public called for stricter regulations than those being proposed, similar to those for tobacco products.
With respect to advertising at points of sale accessible to youth, vape shop owners and vaping industry associations were supportive of the proposed restrictions. They mentioned that several provinces have already implemented restrictions on advertising at retail establishments accessible to youth. However, one vaping industry association commented that a complete prohibition on advertising in public places would have a negative impact on persons who smoke.
Most of the larger manufacturers (multinational manufacturers of both tobacco and vaping products), one vaping association and all retail associations were against the proposed advertising restrictions at points of sale. They stated that the proposed restrictions would limit communication on the availability of vaping products as a less harmful alternative for persons who smoke. They felt strongly that the same restrictions on the placement of advertisements should equally apply to vape shops that prevent youth access, since not doing so puts retailers at a competitive disadvantage in terms of how they can market vaping products.
With respect to websites where vaping products are sold, manufacturers suggested that the proposed regulations should clearly indicate the criteria that Health Canada would use to determine if appropriate measures to prevent youth access were in place. One manufacturer asked whether an age verification box, as is often used on websites for online retailers, would be sufficient or if an age-gate done in combination with a third-party age verification would be required in the proposed Regulations. In the manufacturer’s view, requiring third-party age verification to access a website may have the unintended consequence of preventing customers who smoke from switching to vaping products.
In general, most respondents were supportive of the proposal to restrict advertisements in public places or did not express any opposition to the proposal. Several vape shop owners proposed that broad, national brand-specific advertising campaigns in public venues be prohibited except in age-restricted locations. One manufacturer suggested that all outdoor advertising be prohibited within 500 feet of any schools, youth-oriented facilities and childcare facilities. However, one manufacturer commented that if advertising in public places were to be severely restricted, they should be allowed to communicate at retail establishments with persons who smoke to let them know that vaping products are a less harmful alternative to smoking.
Some vape shop owners mentioned that advertisements of vaping products in broadcast media should be restricted to adult programming. Another suggested that advertisements should only be allowed after prime-time hours. Some vape shop owners felt that the proposed criteria to prohibit promotion during “youth-oriented” programming could be problematic, as it would only capture children’s programs. Instead, they suggested that advertisements only be permitted after a specified time or only during adult viewing times.
Most vaping manufacturers were supportive of the proposal and commented that advertisements must not be directed at young persons. They suggested that no media should display vaping products advertisements if more than 25% of the audience is below 25 years old. They also supported the proposal to prohibit advertisements in children’s and youth-oriented publications, including electronic publications such as websites and social media platforms. However, they expressed concerns that the term “youth-oriented” should be clearly defined. Some also mentioned that the enforcement of such measures in the online domain would be challenging, and therefore one manufacturer suggested that advertising in social media should be completely banned.
Certain vape shop owners mentioned that businesses should be allowed to use signs and billboards to advertise the company name, location, website, phone number and hours of operation. They also indicated that such signs should be allowed to display authorized statements that compare health effects. One vaping industry association mentioned that Facebook and other social media platforms are critically important to ensure that adults who smoke or vape have access to information and support. Several vape shop owners mentioned that communication through social media is important for their business to reach out to adults who smoke.
Health Canada also consulted the public on measures to prohibit the display of vaping products at points of sale. Such restrictions would not apply at points of sale where youth do not have access (e.g. a vape shop that does not allow youth on its premises or that blocks access to its website to youth), as long as the products cannot be seen from the outside of these places.
Most of the larger vaping manufacturers and the retailer associations were strongly against these proposals, stating that such restrictions are not reflective of a balanced approach that recognizes the harm-reduction potential of vaping products compared to tobacco products. Furthermore, they stated that the proposed restrictions would considerably limit communication to persons who smoke about the availability of vaping products as a less harmful alternative to tobacco products, especially at locations where they purchase tobacco products.
In response to the comments received during these consultations, Health Canada deemed that a prohibition on advertising that can be seen or heard by young persons with limited exceptions was the most appropriate approach to protecting youth. The approach does not go as far as implementing tobacco-like restrictions as suggested by some commenters due to the potential of vaping products to be a less harmful alternative to tobacco use. However, in response to concerns about the impact of advertising on youth, strict measures were adopted to maximize youth protection, which allow the vaping industry to implement measures to limit their advertising to adults.
Consultation on health warnings on advertising prior to prepublication in the Canada Gazette, Part I
The 2017 Consultation also sought comments on whether vaping products that contain nicotine should display a health warning on the vaping product or its packaging such as “WARNING: This product contains nicotine. Nicotine is an addictive substance. Use of nicotine during pregnancy may harm the fetus” in English and “MISE EN GARDE : Ce produit contient de la nicotine. La nicotine crée une dépendance. L’usage de la nicotine durant la grossesse peut nuire au fœtus” in French (Proposal 3).
Many of those who responded to the 2017 Consultation supported the display of health warnings, although several suggested additional health warning statements should be developed. Some commenters felt that the health warning statement needed to be stronger to truly warn of harm, while others felt that the proposed health warning might discourage persons who smoke from switching to a less harmful alternative (i.e. vaping). Although the feedback was in response to health warnings on a product or package, Health Canada has considered the comments in the development of health warnings on advertisements.
One of the measures described in the 2019 Consultation was to require that advertisements include a health warning. The content, format, size and manner of display of the health warning would be prescribed by regulations. Where the advertisement only has an audio content, the applicable health warning would have to be read aloud. The example included in the Notice of Intent of a health warning that could apply to vaping products containing nicotine was the following in English and French:
- “Vaping products contain nicotine. Nicotine is highly addictive. Vaping products also release chemicals that can harm your health. Youth and adult non-smokers should not vape. Health Canada”
- “Les produits de vapotage contiennent de la nicotine, une substance qui crée une forte dépendance. Ils libèrent aussi des substances qui peuvent être nocives pour la santé. Les jeunes et les non-fumeurs ne devraient pas vapoter. Santé Canada”
The proposed health warning on nicotine’s addictive properties received widespread support. With regard to the proposed text, several NGOs commented that the proposed wording to the effect that youth and non-users should not vape could solicit interest from adolescents and be counterproductive, and therefore recommended that this wording be removed. There were also suggestions that alternate health warnings be considered, such as health warnings concerning the risk of damage to the developing adolescent brain and the risk of vaping leading to tobacco use.
Numerous vape shop owners and most vaping product manufacturers expressed reservations about the statement “Vaping products also release chemicals that can harm your health.” They mentioned that it is factually ambiguous and could misinform Canadians or discourage persons who smoke from switching to vaping products. Some suggested that the word “can” be replaced by the word “may.”
Several manufacturers and vape shop owners stated that the proposed health warnings should be attributed to Health Canada, a respected source of information.
An association of broadcasters suggested that the health warnings be no longer than 25 words and readable in five seconds or less, to permit reasonable use on audio media, such as the radio. Furthermore, the association commented that Health Canada should consider requiring a generic health warning for the advertising of non-substance specific vaping devices, such as “Vaping devices may release chemicals that can harm your health. Youth should not vape. Health Canada.”
The health warnings selected by Health Canada are based on the findings of the NASEM report, published in 2018. In response to comments on the need to inform Canadians about the harms of vaping and to stay current with the science, the Regulations incorporate by reference the List, which can be amended as needed. This will allow Health Canada to respond to emerging science and technology that may warrant modifying the health warnings. Furthermore, the Regulations give the choice of any health warning from the List to be conveyed in a vaping product advertisement to the industry. This is expected to facilitate industry compliance and simplify enforcement activities by Health Canada.
Public opinion research
In 2018, Health Canada commissioned public opinion research (POR) on nicotine-related health warnings. A summary of this research can be found in the Evaluation of Possible Labelling Elements for Vaping Products — Phase I and Phase II: Final Report, published in April 2018. footnote 9 This POR included testing of the statement “Caution: Nicotine is highly addictive – Health Canada” with two alternate statements: “Warning: This product contains nicotine. Nicotine is an addictive substance” and “This product contains nicotine which is a highly addictive substance – Health Canada.”
Participants felt that the messages — i.e. that the product contains nicotine and that nicotine is highly addictive —should be included in one short, simple statement for added clarity, prefaced by the word “warning.” The Health Canada attribution was considered important to participants to establish credibility.
In November 2018, Health Canada commissioned POR on public perceptions of nicotine. A summary of this research can be found in the final report entitled Qualitative and Quantitative Research on Perceptions of Nicotine, published in March 2019. footnote 10 This POR included the testing of several potential health warnings. The study showed that the statement “Vaping products contain nicotine, a highly addictive substance” in English and “Les produits de vapotage contiennent de la nicotine. La nicotine est une substance qui crée une forte dépendance” in French were preferred, as they were deemed to be simple, clear and factual.
The health warnings were further adjusted as a result of POR and based on feedback received through consultation with the public and stakeholders. The attribution of the health warning to Health Canada and the use of the word “WARNING” preceding the text of the prescribed health warning was included in the requirements as a result of the POR findings that suggested that these changes would improve their effectiveness.
Prepublication in the Canada Gazette, Part I
The proposed Regulations were prepublished on December 21, 2019, in the Canada Gazette, Part I (CGI), for a 30-day consultation period that ended on January 20, 2020.
A total of 242 responses were received including 37 from the vaping industry (includes 13 manufacturers of vaping products, 24 vape shop owners), 1 convenience store, 8 industry associations, 103 from the general public (includes 21 vapers and 10 former smokers), 12 non-governmental organizations, 25 public health authorities, 31 health care providers, 10 associations of health professionals, 3 provincial governments, 5 from academia and 7 from other sources. All comments were reviewed and taken into consideration when finalizing the Regulations.
Many submissions were out of scope for this regulatory initiative. Health Canada has examined these submissions and will use them in the development of future measures regarding vaping products.
Part 1 — Advertising and Point of Sale Promotion
Scope of the prohibition on advertisement
Several associations of health professionals and public health authorities indicated that Health Canada has not considered young adults and non-users of tobacco in the design of Part 1 of the Regulations. They suggested that young adults, described as up to the age of 25 or 29 years old, should also be within the scope of application of the prohibition.
Response: The objective of the current Regulations is to protect young persons from inducements to use vaping products, which in turn helps address the rapid rise in youth uptake of vaping. Additional promotion restrictions may also be considered in the future. A young person is defined in the TVPA as a person under eighteen years of age. For adults, including young adults who may be exposed to advertisements, the Regulations require a health warning be conveyed on all vaping advertisements to warn them about the harms of vaping product use.
Unfair treatment compared to other products
Large manufacturers, industry associations of retailers or convenience stores were strongly against the prohibition on advertising and promotion at retail establishments where youth have access. They claimed that this measure was unfair given their proven track record of keeping age-restricted products, such as tobacco products, out of the hands of minors. Vape store owners who operate an online point of sale were also opposed to measures that prohibit advertising online to which youth have access. They mentioned that such a prohibition does not exist for cannabis, alcohol, tobacco or sexually explicit material.
Response: Health Canada believes that the Regulations are proportionate to the issue at hand, that is the rapid rise in youth vaping. Contrary to the claim made in the stakeholders’ comment, the TVPA does not allow tobacco products to be advertised to youth online. The Cannabis Act requires that regulated entities take reasonable steps to ensure that cannabis promotion that is communicated by means of telecommunication cannot be accessed by young persons.
Part 2 — Required Information in Advertising (Health Warning)
Vaping products without nicotine
It was suggested that advertisements of products without nicotine do not need to convey any health warning, or that advertisements of vaping devices and their parts should be exempt from the requirement to convey a health warning. Other contrasting comments suggested that both health warnings should be conveyed on all advertisements of vaping products.
Response: All advertisements of vaping products, devices and their parts, including those that do not contain nicotine, are required to convey one health warning from the List. Vaping products, including those without nicotine, are harmful. They emit an aerosol that contains potentially harmful chemicals. The inhalation of these emissions into the lungs may have a negative impact on health (e.g. lung damage). There are no specific conditions that require a particular prescribed health warning be used on an advertisement of a vaping product or a vaping product-related brand element. Manufacturers, importers and retailers could also alternate the prescribed health warnings on their advertisements or choose to display other warnings in addition to the prescribed health warnings.
Content of the health warning
The following health warnings were proposed in the Canada Gazette, Part I, for inclusion on the initial list:
- Warning 1: WARNING: Vaping products contain nicotine, a highly addictive chemical.
- Warning 2: WARNING: Vaping products release chemicals that may harm your health.
There was general agreement with Warning 1. However, Warning 2 was not well received by the associations of health professionals, public health authorities and NGOs. Alternate wording on the harms and health effects of vaping, or on specific health effects (i.e. damage to the lungs, cardiovascular system, etc.) were offered for consideration. Vape shop owners suggested that the health warning include a statement that youth and non-smokers should not use these products. Some manufacturers of vaping products and certain vape shop owners commented that the health warning should also state that vaping is less harmful than smoking.
Response: In regards to comments that alternate wording for Warning 2 be considered about the harms and health effects of vaping, they will be considered for future amendments to the List. The modification of the existing health warnings and development of new health warnings involves extensive research including literature reviews, reviews by experts in the field to ascertain the accuracy of the statements, as well as public opinion research to determine whether the health warnings are understandable, informative, believable and credible. Health Canada will continue to monitor the emerging evidence on the harms associated with vaping products, which will inform the introduction of new health warnings or the removal of existing health warnings as necessary and appropriate. The Regulations provide the flexibility to modify the List.
The suggestion that the health warning include the statement that youth and non-smokers not use these products was not retained by Health Canada as the research shows that such statements have the opposite effect on youth and leads them on to try “forbidden” products, which is consistent with risky behaviours or attitudes observed among adolescents.
Presentation of the health warning
Comments from public health authorities and NGOs indicated that the health warnings should be increased to cover 50% or more of the surface area of the advertisement, and that they should include colours, graphic images and be rotated, similar to what is in place for packages of tobacco products. It was also suggested that a quit line or website for cessation services be included in the health warning.
Response: Health Canada will take these suggestions under consideration in developing new requirements for health warnings in vaping advertising that align with those on tobacco packaging for future modifications to these Regulations. Further research and analysis could be conducted to support the development of such measures.
Official language requirements for health warnings
Several public health authorities and NGOs suggested that the health warning be only displayed in either English or French, in the language of the advertisement. They reasoned that making this an explicit requirement rather than allowing an exemption from the requirement to convey the health warning in both official languages would improve the legibility of the health warning.
Response: Health Canada has made changes to the official language requirements. The Regulations require that the health warning be conveyed in both official languages if the advertisement is in both official languages or in a language other than the official languages. However, if the advertisement is only in one official language or in an official language and another language, the health warning must be conveyed only in the official language of the advertisement.
Other concerns
Coming into force
A number of vaping industry respondents commented that the proposed coming into force timelines for the requirements in the proposed Regulations were too short, and an extended period would give them more time to comply with the requirements. In contrast, NGOs called for the requirements to come into force as soon as possible after adoption of the Regulations to protect youth.
Response: The timelines for coming into force in the proposed Regulations that were prepublished in CGI have been maintained. This means the advertising restrictions set out in Part 1 come into force 30 days after publication of the Regulations, except for restrictions on the display of vaping products and their packaging at points of sale which come into force 60 days after publication of the Regulations. The measures in Part 2 with regard to conveying a health warning in advertisements come into force 30 days after publication of the Regulations. The timelines provide sufficient time for the vaping industry to comply with the requirements. Extending the implementation period would have resulted in young persons being exposed to vaping product advertising for a longer period of time, thus undermining the objectives of these Regulations.
Regulating vaping products in the same manner as tobacco products
Numerous stakeholders, primarily public health authorities, provinces, NGOs and some members of the public have called for vaping products to be subject to tobacco-like restrictions, e.g. by prohibiting all advertising of vaping products with certain exceptions.
Response: The restrictions on vaping promotion set out in these Regulations bring the overall vaping promotion regime much closer to that in place for tobacco products. The Regulations also reflect the TVPA’s legislative approach to regulating vaping products as a separate product category from tobacco products. As well, the Regulations are aligned with the TVPA’s objectives specific to vaping products, particularly the objective of protecting young persons and non-tobacco users from inducements to use vaping products. The Regulations leave room for vaping product promotion and advertising that is compliant with the Act and regulations to continue to reach adults. This takes into account the best and current available scientific evidence that suggests that, although they are harmful, vaping products are less harmful than cigarettes for people who switch completely from smoking to vaping.
Statements comparing health effects
Several manufacturers and vape shop owners have reiterated their calls for Health Canada to authorize statements that compare the health effects of vaping to those of smoking. Some even suggested that health warnings be accompanied by authorized statements that compare the health effects of vaping to those of smoking. There was a concern that without such statements, adult smokers would not be aware of the potential benefits of completely switching to vaping.
Response: The objective of the current Regulations is to address the rapid increase in vaping product use by young persons and to warn about the harms of vaping product use. Additional regulatory measures, such as authorizing statements comparing the health effects of tobacco products and vaping products are still under consideration. In the meantime, manufacturers of vaping products who want to make a health claim must obtain the required authorizations pursuant to the Food and Drugs Act (FDA) before the products can be commercially imported, advertised or sold in Canada.
Online age-gating and privacy concerns
Concerns were raised regarding the protection of customers’ personal information in connection with putting in place an age-gate mechanism online in an effort to comply with the new requirements to prevent young persons from being exposed to online vaping products related advertising. Concerns that such measures would be costly and would limit global competitiveness were also raised by vape shop owners and industry associations.
Response: The Personal Information Protection and Electronic Documents Act sets out the basic rules for collection, use or disclosure of personal information by private sector companies in the course of their business activities in Canada. More information can be obtained from the Office of the Privacy Commissioner of Canada’s website, and particularly in the Guidelines for Identification and Authentication. The Regulations do not specify a particular age-gate mechanism that must be implemented. Regulated parties must implement the most appropriate and effective measures that prevent youth access to their websites or social media pages which may require the collection of personal information to verify age. In addition, they are also responsible for taking all necessary steps to comply with other statutory and regulatory requirements that may apply, such as those relating to the protection of personal information.
Other non-regulatory actions to address youth vaping
Non-regulatory action such as ramping up public education campaigns was also suggested by stakeholders to address youth vaping.
Response: Health Canada has launched a youth-oriented public education campaign with national reach to increase awareness about the harms and risks associated with vaping product use among youth. In addition, grants and contributions funding of $14 million have been allocated over four years to address tobacco use and youth vaping through the Substance Use and Addictions Program. New investments are being made for additional research and surveillance activities. Health Canada continues to work with its provincial and territorial counterparts in areas of shared jurisdiction.
Modern treaty obligations and Indigenous engagement and consultation
The Regulations are not expected to impact modern treaties with the Indigenous peoples of Canada. Analysis regarding possible differential impacts on Indigenous persons is set out in the “Gender-based analysis plus (GBA+)” section below.
Instrument choice
Option 1: Baseline scenario
This option would consist of enforcing the existing legislative regime with respect to promotion and would not include the adoption of further federal restrictions on vaping product promotions through regulations. The TVPA implemented a tailored approach to vaping product promotion to protect youth and non-users of tobacco products. While certain types of vaping product promotion known to be particularly effective on youth and non-users of tobacco products were prohibited in the TVPA, other forms of vaping product promotion (e.g. information and brand preference advertising) are still permitted but are subject to additional restrictions that may be imposed by regulations in the future, if needed, to respond to emerging evidence and continue to protect young persons and non-users of tobacco products from inducements to use vaping products. Option 1 would not implement additional limits on vaping product promotion to those already present in the TVPA. Young persons would continue to be exposed to vaping product promotion that currently appears in public spaces including at points of sale, where vaping products and their packaging are displayed and visible to everyone, including youth.
This option would leave youth exposed to a considerable amount of vaping product promotion, since advertising could continue to appear in public places, including online, in any publications and broadcast media, and in any locations that are not subject to provincial and territorial restrictions on vaping promotion. This option would not respond to new scientific evidence that indicate that young persons who are exposed to vaping product promotion including information and brand preference advertisements and product displays are more likely to try and to use vaping products regularly.
Therefore, the status quo is not considered to be an appropriate option.
Option 2: Partial restrictions on vaping promotions as proposed in the 2019 Consultation
This option would consist of new regulatory measures to restrict the placement of advertising of vaping products in certain specific places, such as in parks or near schools, or media, such as social media, that are associated with young persons. This option would also prohibit the display of vaping products at retail establishments accessible to youth, limit the content of advertising and require a health warning on all advertisements. This approach was outlined in the Notice of Intent consultation that was launched in February 2019.
Under this option, there would be a reduction of advertising that could induce youth to use vaping products, but youth could still be exposed to vaping product promotions inadvertently through broadcast media at certain times, in certain public places and in print publications, including online, even if the media, places and publications do not contain any youth-oriented material. Also, the definition of youth-oriented programming or publication was considered, but was found to be too subjective for regulated parties to apply and for Health Canada to enforce.
This option would not prevent vaping product advertising in most public places or in publications that could still be seen or accessed by youth.
Option 3: Recommended option — Vaping Products Promotion Regulations
This option would generally prohibit all vaping product advertising that can be seen or heard by youth. Certain forms of advertising that have limited potential to be seen or heard by young persons would be exempted from this prohibition. The Regulations do not prohibit vaping product promotions in publications that are addressed and sent to a named adult and publications that are provided to adults on request at a retail establishment. Signs at the point of sale indicating only the availability and price of vaping products that meet the form and placement conditions set out in the Regulations would also be permitted.
Advertising and display of vaping products in places where young persons do not have access, such as vape shops, bars, casinos and online publications that are not accessible to young persons, would remain permitted as long as it complies with the requirements of the TVPA and conveys the required health warning statement. Adult access to such vaping product promotion would be therefore maintained.
All vaping advertisements (other than signs at points of sale that indicate the availability and price in the prescribed form) would have to convey a health warning. These measures would support the TVPA objective of enhancing public awareness of the health hazards of using vaping products.
This option was chosen since it is the most effective approach to support the objective of protecting youth from inducements to use vaping products as it responds to new evidence that demonstrates that youth exposed to vaping product advertisements exhibit a greater openness to try and a greater likelihood of using vaping products. This option also leaves room for the vaping product industry to continue to communicate with adults with regard to vaping products, including through commercial promotion.
Benefits and costs
The Regulations would result in total incremental costs estimated at $7.7 million present value (PV) over 10 years (or $1.1 million annually), consisting of $4.2 million (or $0.6 million annually) in industry costs and $3.5 million (or $0.5 million annually) in government costs. The monetized costs to industry are associated with complying with the prohibition on the display of vaping products at points of sale where they can be seen by youth as well as, the requirement that a health warning be conveyed in advertisements of vaping products. The government costs are associated with the costs to monitor and enforce compliance with the Regulations.
Due to data limitations, there are other costs that could not be monetized and were therefore analyzed qualitatively. These qualitative costs include costs of implementing measures to prevent youth from being exposed to advertising, costs of future changes to the List, the potential profit losses for the vaping and the advertising industries, and the impact on adults who smoke who will now be exposed to less advertising inciting them to use vaping products as an alternative to tobacco products.
Despite the costs of the Regulations, the total benefits are expected to outweigh the total costs. The Regulations support the CTS that aims to reduce the burden of disease and death that results from tobacco use including its impact on the public health system. The Regulations are expected to primarily benefit youth by contributing to a reduction in the number of youth transitioning into lifelong tobacco users. The Regulations (i) restrict vaping product promotions to youth in order to protect them from inducements to use vaping products which exposes them to nicotine and an increased risk of tobacco use and (ii) enhance public awareness about the health hazards of using vaping products that will enable adults to make an informed choice regarding the use of these products, including avoiding exposure to nicotine. Long-term economic benefits will be realized in terms of avoided tobacco-related mortality and morbidity, and exposure to second-hand smoke. Even if they cannot be directly quantified, the benefits are expected to outweigh the costs of the Regulations.
Analytical approach
The Cabinet Directive on Regulation requires departments to analyze the costs and benefits of federal regulations. To measure these impacts, the benefits and costs are estimated by comparing the incremental change from the current regulatory framework (i.e. the “baseline scenario”) to what is anticipated to occur under the new regulatory approach (i.e. the “regulatory scenario”). The Regulations will come into effect in 2020. The period of analysis for this cost-benefit analysis (CBA) covers the 10-year period from 2020 to 2029. As per Treasury Board of Canada Secretariat guidelines, footnote 11 the CBA only assesses incremental impacts directly related to a regulatory requirement. A 7% discount rate is used to estimate the present value of the incremental costs and incremental benefits. All values reported for the 10-year period are expressed in 2019 constant dollars.
The regulatory impacts in this analysis have been estimated using two approaches: quantitative analysis, where possible, and qualitative analysis. A copy of the full CBA report is available upon request from hc.pregs.sc@canada.ca.
Impacts in the CBA have changed since prepublication in CGI due to new information and updated data. The following is a description of these changes:
- The number of impacted vape shops, G&C stores, manufacturers, importers and online stores were updated with more recent data collected by Health Canada on the vaping market.
- An additional cost to vape shops to apply health warning stickers on vaping product brochures supplied by manufacturers and importers was added. It is assumed that vape shops will apply health warning stickers on product brochures to comply with the Regulations, at least initially until new product brochures are printed by their suppliers.
- Additional qualitative impacts on vaping companies that plan on continuing to advertise either in public places or by means of telecommunication were included.
Overview of the vaping industry in Canada
According to a 2019 market study, the vaping product market in Canada was estimated to be approximately $1.36 billion. Vaping products are sold in three main categories of stores: vape shops (49%), online (21%) and G&C stores, grocery stores, drugstores and mass merchandisers (30%). It was estimated that there were around 1,400 vape shops in 2019. Of that number, 350 (25%) are likely large businesses. Based on 2016 data, it is estimated that there are 27,240 G&C stores, of which 63% are considered small business. footnote 12, footnote 13 Furthermore, there were approximately 1,500 websites for vaping products. Most of them were the online retail component of brick and mortar vape shops.
Overview of vaping product users in Canada
According to the 2017 Canadian Tobacco, Alcohol and Drugs Survey, 3% (863,000) of Canadians aged 15 years and older reported using an e-cigarette in the past 30 days. Among these users, 65% (557,000) were current smokers, 20% (173,000) were former smokers and 15% (133,000) were never smokers. Of these never smokers, 58% (77,000) were youth aged 15 to 19 and 33% (45,000) were young adults aged 20 to 24.
Assessment of costs and benefits
It is anticipated that the Regulations will impact the vaping industry, the advertising industry, people who smoke or vape and Health Canada.
Part 1 — Advertising and point of sale promotion
Nine provinces and two territories, namely British Columbia, Manitoba, Saskatchewan, Ontario, Quebec, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, Northwest Territories and Yukon have legislation in effect related to vaping products that include restrictions on the advertising and the display of vaping products at retail establishments.
British Columbia, New Brunswick, Nova Scotia, Newfoundland and Labrador and Yukon do not restrict the placement of advertising in public places. Saskatchewan, Manitoba, Ontario, Prince Edward Island, Northwest Territories have partial restrictions on the placement of advertising in public places. Quebec prohibits advertising in public places.
Alberta and Nunavut have no provincial legislation in place for vaping products and are considered to be most impacted by the Regulations. The Regulations will also impact all online businesses across Canada (with the exception of Quebec where online sale of vaping products is prohibited).
Part 2 — Required Information in Advertising (Health Warning)
The Regulations will impact all businesses, including online businesses across Canada (with the exception of Quebec).
The costs associated with the Regulations are described below.
Quantitative costs of the Regulations
Costs associated with Part 1 of the Regulations (Advertising and Point of Sale Promotion)
Vaping industry costs — Gas and convenience stores
1. Costs to G&C stores to remove vaping products from the view of young persons by purchasing and installing cabinets
In the baseline, the requirement of removing vaping products from the sight of young persons in G&C stores where youth have access is already in place in many provinces across Canada. However, the G&C stores in Alberta and Nunavut would be impacted by the Regulations and may incur incremental costs. Most of these G&C stores already have closed cabinets behind the counter that are used to store tobacco products to comply with provincial and territorial tobacco legislation. G&C store operators can use these cabinets to store their vaping products out of sight in order to comply with the Regulations. Where there is no room in cabinets, it is assumed that retailers will use space under the counter. If the space is limited under the counter, it is expected that the retailers would keep a limited supply in closed cabinets and the rest in their storage room as they do for other products.
It is to be noted that industry-reported cigarette sales in Canada have declined by 24% from 2013 to 2019. footnote 14 The decline has occurred in all provinces and is well distributed across the country. It is assumed that the G&C stores in Alberta and Nunavut would very likely use their existing tobacco cabinets to store vaping products due to space becoming available as a result of the declining sales of tobacco products.
Nevertheless, as a conservative estimate for the purposes of the analysis, it is assumed that 5% of the G&C stores located in Alberta and Nunavut would purchase and install new cabinets in order to comply with the Regulations. There are an estimated 2,758 G&C stores in these jurisdictions. It is assumed that the purchase cost of a cabinet is $1,000, including shipping costs. The installation cost is estimated at $200. It is anticipated that the impacted G&C stores would incur incremental costs associated with purchasing and installing closed cabinets to remove vaping products from the sight of young persons. This cost is assumed to occur in 2020. The total incremental cost is estimated at $165,480 PV.
Costs associated with Part 2 of the Regulations (Required Information in Advertising [Health Warning])
2. Costs of adding a health warning on posters and product catalogues — Vape shops
In the baseline, Quebec already requires the display of health warning statements on advertisements of vaping products. Businesses in Quebec would not be impacted by the Regulations. Of the 1,400 vape shops estimated in Canada in 2019, 420 of them were located in Quebec. footnote 15, footnote 16 All vape shops sell vaping products supplied by other manufacturers and importers. However, some vape shops also sell their own vaping products.
Vape shops that have their own vaping liquids brands generally advertise them by making their own indoor posters and product catalogues. Once the Regulations come into force, it is anticipated that these vape shops would incur incremental costs associated with putting a health warning on in-store posters and product catalogues. Health Canada does not specify how the warning statement is to be added on the product catalogue. It is assumed that the above-mentioned vape shops will comply with the requirements in the most cost-effective way by applying a sticker on their existing stock of product catalogues. These vape shops will also need to have a health warning added to their professionally designed, laminated wall-sized posters.
It is estimated that 30% of vape shops advertise their own vaping products by using indoor posters and catalogues. Those vape shops would incur one-time incremental costs associated with applying a sticker on product catalogues and updating indoor posters for the first time. When the product catalogues and in-store posters need to be reproduced next time, there would be no incremental costs as the vape shops would have already incorporated the required warning in the catalogues and in-store posters. It is assumed that the average price of commissioning a professionally designed, high quality and wall-sized poster is $400, there will be three indoor posters and three product catalogues in each store and the average price of designing and printing of one sticker is $5. The one-time incremental cost associated with acquiring indoor posters with a warning statement is estimated at $352,800 PV, while the one-time incremental cost associated with applying a health warning statement sticker to the product catalogue is estimated at $4,410 PV. The total incremental costs associated with adding a health warning for vape shops that advertise their own products are estimated at $357,210 PV.
It is reasonable to assume that all vape shops sell vaping products supplied by manufacturers and importers therefore also use product catalogues supplied by them. Once the Regulations come into force, it is anticipated that these vape shops would incur incremental costs associated with adding health warning stickers on these product catalogues. The one-time incremental cost associated with applying a health warning statement sticker to product catalogues is estimated at $14,700 PV.
The total incremental costs associated with including health warnings on posters and catalogues for all vape shops are estimated at $371,910 PV.
3. Costs of designing and printing posters and brochures with the health warning — Manufacturers and importers
Large manufacturers and importers provide posters and brochures to vape shops and G&C stores that sell their vaping products. Once the Regulations come into force, manufacturers and importers will incur incremental costs as a result of adding a health warning to brochures and indoor posters.
It is assumed that the price of designing a brochure is $600 and that manufacturers and importers provide approximately 300 brochures to each vape shop and G&C stores selling their products. The one-time design cost to the 200 manufacturers and 20 importers is thus $132,000. The cost of printing these brochures is estimated at $206,232. The one-time incremental cost associated with designing and printing brochures is estimated at $338,232 PV.
Manufacturers and importers would assume a cost to provide an estimated three posters each to 980 vape shops (all vape shops excluding 420 in Quebec) to replace existing materials at the same cost of $400 per poster. The one-time incremental cost associated with designing and printing indoor posters with the health warning for vape shops is estimated at $1,176,000 PV. In total, the incremental costs for the manufacturers and importers to add a health warning on posters and brochures is estimated at $1,514,232 PV.
4. Cost of website design to include health warning — Businesses that advertise vaping products on their websites
Businesses will be required to display a health warning on their websites that promote or sell vaping products. As of December 2019, there were approximately 1,500 online businesses across Canada, including Quebec. However, Quebec prohibits online advertising and sale of vaping products. The Regulations impose specific requirements in the presentation of a health warning on websites. Those online businesses (1,080) will need to redesign their websites in order to include the health warning as per the requirements set out in the Regulations; therefore, they will incur incremental costs.
It is assumed that the average cost of website design is $2,000 per business. It is estimated that the one-time incremental cost associated with including a health warning on websites will be $2,160,000 PV.
Government costs — Health Canada
5. Costs of implementation, enforcement and compliance activities
Implementation of the Regulations will require an investment of public sector resources. In particular, Health Canada will incur incremental costs for implementation activities and to monitor and enforce compliance. Estimates for these costs are described below.
These activities include development of compliance and enforcement (C&E) policy and procedures for the Regulations; development of C&E assessment tools; warning letter templates; preparation of fact sheets (for external distribution) and internal guidelines and compliance promotion plans; and development of training and associated training materials and training delivery to inspection staff.
Health Canada’s baseline expenditures with respect to compliance promotion with the TVPA, which include both personnel and other costs, are likely to change under the Regulations. The additional costs are measured based on the opportunity costs of employee resources. These include time spent by staff informing industry and instructing Health Canada inspectors about the new requirements, as well as the cost of developing and distributing fact sheets and other guidance documents that describe the new requirements. Additional outreach activities may be conducted during the first two years of implementation.
It is expected that the Government of Canada (Health Canada) will incur compliance monitoring and enforcement costs for the following: training of inspectors, ongoing site inspection and travel, analysis of advertisements, investigations into alleged violations, and measures to deal with cases of non-compliance. The initial implementation costs are estimated at approximately $327,000 PV. These are to be coupled with ongoing outreach and compliance and enforcement costs of approximately $3,156,855 PV over 10 years. The total government resource costs are thus estimated at $3.5 million PV over 10 years (or $0.5 million annually).
Qualitative costs of the Regulations
When the Regulations come into force, vaping product promotions will be prohibited where they could be seen or heard by youth with specific exceptions. The cost impacts are explained below.
6. Cost of implementing measures to prevent youth from being exposed to advertising in public places and online
The Regulations do not specify the measures that vaping companies must put in place to ensure that they prevent young persons from seeing or hearing vaping advertising in public places including retail establishments or by means of telecommunication including at points of sale online.
In public places where vaping product advertising can be seen or heard by young persons, including retail establishments that permit youth access, there would be a one-time cost related to the removal of the advertising material, which may range from costs related to taking down posters to removing large billboards that promote vaping products. The Regulations provide exceptions at retail establishments that permit youth access to display signs that only indicate the availability and price of vaping products and permit advertising by means of a publication that is only made available upon request by an adult. Most vape shops already prevent young persons from entering their retail establishments and therefore would not incur any additional costs.
For advertising by means of telecommunication, including at online points of sale, there are a variety of measures that the vaping industry could implement to comply with the Regulations. These include, but are not limited to, removing their online advertising; redesigning point of sale websites to remove advertising but retaining information about the availability and price of vaping products; implementing appropriate age-gating mechanisms that verify the age and identity of visitors that access the website. Various age-gating mechanisms can be employed including requiring government issued identification at the point of entry of a website, through the use of third party verification technology, or by providing a unique access code to a person whose age and identity were previously verified prior to being provided an online access code.
The costs related to comply with the requirements of the Regulations may include website redesign costs to remove online advertising and product displays should online retailers choose not to employ age-gating mechanisms. If an age-gating mechanism is employed, the cost will depend on the mechanism chosen, with some third party verification companies charging fees that are based on a fee per transaction or subscription-based services that cost from $25 to several hundred dollars a month according to prices advertised online by these service providers. Health Canada does not have the data necessary to quantify these costs due to the variety of measures and technologies that can be used to comply with Regulations. It is expected that regulated parties will implement the most cost-effective measures to comply with the Regulations.
7. Cost of future changes to the List — All vape shops, manufacturers, importers and businesses that advertise their vaping products on their websites
The List that is incorporated by reference in the Regulations may be amended by Health Canada from time to time to reflect new scientific research on the health effects or health hazards of using vaping products. The document can be updated with additional health warnings, the removal of existing ones or amendments to the text of existing health warnings.
Not all the future changes to the List will impose cost impacts on affected stakeholders. For example, the addition of new health warnings to the List will not result in costs to affected businesses. Businesses can continue to use their existing advertising materials (brochures, catalogues, indoor posters, etc.) or websites and choose to incorporate the new health warning when they update or produce new advertising material.
However, if a health warning on the List is replaced with a new or modified health warning, all vape shops, manufacturers, importers and businesses that use the health warning that was replaced or modified would be impacted and will incur costs to replace the warning statement on their promotional material. However, Health Canada does not have a schedule that outlines when or how often the List will be amended in the 10-year analytical period. Such changes will be determined by the evolving science around vaping product harms.
Regulated parties and interested stakeholders will be notified of any proposed changes to the List. The Regulations allow for a 60-day transition period following any change to the List to allow for the phasing out of advertisements conveying modified or deleted health warnings. Hence, businesses would be able to align their advertising stock lifecycle to minimize the potential costs. It is acknowledged that these future changes may impose costs; however, it is not anticipated that these costs will be significant given the outreach Health Canada plans in advance of changes and based on the lifecycle of promotional materials.
8. Potential profit loss — Retail industry, vaping industry and advertising industry
Vaping product advertising will be prohibited at points of sale, in publications, online, in broadcast media (e.g. TV and radio) and on social media platforms that are accessible to youth. This measure will impact businesses in the vaping industry in all provinces and territories except Quebec, which already prohibits these forms of promotion. The restrictions, which are meant to protect youth, may also result in fewer adults being exposed to vaping advertisements which could lead to a loss of profits to the vaping industry due to reduced sales.
a. Retail industry — G&C stores
The Regulations also restrict vaping product advertising, including their display at points of sale that are accessible by youth. It is anticipated that there will be some incremental revenue impacts on G&C stores where provincial legislation does not already restrict vaping promotions at retail. Thus, vaping product promotion at retail establishments in Alberta, and Nunavut will be restricted when the Regulations are implemented. These restrictions may result in a reduction in the sale of vaping products. The impact may be minimized as the Regulations create exceptions that permit the display of signs about the availability and price of vaping products and permit advertising in brochures that can be made available to adults by request. Health Canada does not have the data necessary to quantify this cost at this time.
b. Vaping industry — Manufacturers and importers
Vaping product manufacturers and importers will no longer be permitted to advertise in broadcast media where youth have access. However, many of these manufacturers and importers also sell their products through vape shops that do not permit youth access, which will continue to be allowed to advertise vaping products to adults, and will therefore not be impacted by the Regulations. It is anticipated that the vaping product manufacturers and importers who sell their products through G&C stores may experience some profit loss as a result of the Regulations. Health Canada does not have the data necessary to quantify this cost at this time.
c. Advertising industry
Canada already has a strong regulatory framework for vaping products in place through the TVPA, which prohibits, among other things, advertising that appeals to youth. Vaping product advertising is a relatively small segment in the advertising industry in Canada. However, vaping product advertising that can be seen or heard by youth would be prohibited, while exceptions to allow the advertising of vaping products to adults would be maintained although in more limited forms. It is anticipated that advertising agencies employed by the vaping industry might experience some profit loss, as this stream of business will be impacted as a result of the restrictions on advertising. Health Canada does not have the data necessary to quantify this cost at this time.
9. Potential cost impact on adults who smoke
Advertising restrictions may reduce awareness of vaping products that are available on the market among adults who smoke. This may result in fewer of them making a switch to a less harmful alternative than cigarettes. However, this will be mitigated by the continued in-store display of signs that indicate the price and availability of vaping products and in-store brochures available to adults upon request. Adults will also continue to have access to vaping product promotion and other information about vaping products in places and mediums (e.g. vape shops and adult only websites) only accessible to adults. Furthermore, non-promotional information on vaping products will remain accessible to the public on the Government of Canada website, in publications by researchers and other academic institutions and in publications by health authorities and NGOs.
10. Impact on competitiveness — G&C stores
Except for advertisements that are limited to signs on availability and price and brochures that can be provided to adults on request, G&C stores would lose most of their ability to proactively advertise or promote vaping products.
However, G&C stores sell types and brands of vaping products that are different from those available at vape shops. footnote 17, footnote 18 The vaping products sold in G&C stores are mostly from large tobacco manufacturers who have a long-standing and well-established business relationship with these retailers. These large tobacco manufacturers have promoted their products inside G&C stores and outside the retail environment, where they are not prohibited by provincial legislation. G&C stores also sell tobacco products to individuals who are most likely to also purchase vaping products.
The advertising and display ban at retail establishments will impact the ability of new entrants to gain vaping product market share through the G&C stores channel. New market entrants may prefer vape shops and online channels for product launches, given a greater ability to display and advertise vaping products to adults in those spaces. The current dominant companies might also increase their efforts to sell their products through vape shops that prevent youth access, or step up efforts to open their own brand-specific vape stores that prevent youth access. Hence, it is expected that the Regulations could have a negative impact on the competitiveness of G&C stores in this product category if the current business model continues to operate in the future.
Benefits of the Regulations
1. Benefits associated with Part 1 — Advertising and Point of Sale Promotion
Part 1 of the Regulations restricts vaping product promotions to youth in order to protect them from inducements to use vaping products which exposes them to nicotine and an increased risk of tobacco use. In addition, in an environment where commercial advertising of vaping products is restricted, the Regulations will help increase the effectiveness of Health Canada’s or the provinces’ education campaigns that inform youth and non-users of tobacco products about the harms of vaping.
2. Benefits associated with Part 2 — Required Information in Advertising
Part 2 of the Regulations enhances public awareness about the health hazards of using vaping products that will enable adults to make an informed choice regarding the use of these products, including avoiding exposure to nicotine. Furthermore, adults who are also parents or mentors of young persons will be informed about the harms of using vaping products, which will enable them to reinforce the messaging about the harms of vaping products to young persons.
3. Public health benefits
Successful implementation of the CTS depends on strong collaboration and coordinated efforts. There is ongoing cooperation between the federal government, the provinces and territories, municipalities, non-governmental organizations, community agencies and the private sector. The combined efforts of many groups help support tobacco control gains across the country, which have resulted in a significant decline in tobacco-attributable mortality and morbidity, in turn yielding long-term economic benefits.
The Regulations support the CTS that aims to reduce the burden of disease and death of tobacco use including its impact on the public health system. The Regulations are expected to primarily benefit youth by contributing to the reduction in the number of youth transitioning into lifelong tobacco users. Long-term economic benefits will be realized in terms of avoided tobacco-related mortality and morbidity, and exposure to second-hand smoke. Even if they cannot be directly quantified, the benefits are expected to outweigh the costs of the Regulations.
Summary
The Regulations are estimated to result in a total incremental cost of $7.7 million PV over the 10-year period (or $1.1 million annually). The public health benefits resulting from the Regulations, including the potential benefit of protecting young people from inducements to use vaping products, are considerable, even if they cannot be directly quantified. It is expected that these benefits will outweigh the costs of the Regulations. The following table provides a detailed cost-benefit statement.
Base Year |
Year 4 |
Year 7 |
Year 10 |
Total PV |
Annualized Average |
|
|---|---|---|---|---|---|---|
A. Quantified impacts (2019 constant dollars, CAN$) |
||||||
Costs |
||||||
Costs to G&C stores |
||||||
Purchase and installation of cabinets |
165,480 |
0 |
0 |
0 |
165,480 |
23,561 |
Costs to vape shops that advertise their own products |
||||||
Updating indoor posters — health warning |
352,800 |
0 |
0 |
0 |
352,800 |
50,231 |
Apply stickers to product catalogues — health warning |
4,410 |
0 |
0 |
0 |
4,410 |
628 |
Costs to vape shops that advertise products supplied by other manufacturers and importers |
||||||
Apply sticker to product catalogues — health warning |
14,700 |
0 |
0 |
0 |
14,700 |
2,093 |
Costs to manufacturers and importers that provide brochures and posters to vape shops and G&C stores |
||||||
Produce new brochures — health warning |
338,232 |
0 |
0 |
0 |
338,232 |
48,157 |
Update new posters — health warning |
1,176,000 |
0 |
0 |
0 |
1,176,000 |
167,436 |
Costs to businesses that advertise vaping products on their websites |
||||||
Web design — health warning |
2,160,000 |
0 |
0 |
0 |
2,160,000 |
307,535 |
Total industry costs |
4,211,622 |
0 |
0 |
0 |
4,211,622 |
599,640 |
Costs to government |
||||||
Implementation |
327,000 |
0 |
0 |
0 |
327,000 |
46,557 |
Compliance and enforcement |
225,000 |
450,000 |
450,000 |
450,000 |
3,156,855 |
449,465 |
Total government costs |
552,000 |
450,000 |
450,000 |
450,000 |
3,483,855 |
496,023 |
Total costs of Regulations |
4,763,622 |
450,000 |
450,000 |
450,000 |
7,695,476 |
1,095,663 |
Costs to small businesses |
3,543,384 |
0 |
0 |
0 |
3,567,608 |
507,947 |
B. Qualitative impacts |
||||||
Benefits |
||||||
1. Increased protection of youth from inducements to use vaping products leading to fewer of them initiating the use of vaping products that would expose them to nicotine and an increased risk of tobacco use. 2. Enhanced public awareness of the health hazards of using vaping products that would enable Canadians to make an informed choice on the use of these products, including avoiding one’s exposure to nicotine.. 3. Supports CTS that aims to reduce the burden of disease and death from tobacco use and its impact on the public health system. |
||||||
Costs |
||||||
1. Businesses in the vaping industry, advertising industry and retail industry in all provinces and territories except Quebec would be negatively impacted as a result of loss of profits or revenue. 2. Businesses that advertise vaping products would be impacted due to costs of implementing measures to comply with the prohibition on advertising that can be seen or heard by young persons. 3. G&C stores would lose most of their ability to proactively advertise or promote vaping products and this would have a negative impact on their competitiveness with regard to vaping products sales. The market share of G&C stores may be negatively impacted as new entrants may promote their products at other retail outlets such as vape shops or online. 4. Adults who smoke may become less aware of vaping products that are available on the market. This may result in fewer of them making the switch. However, the continued in-store display of signs indicating the price and availability of vaping products and brochures available to adults upon request and the availability of all other non-promotional sources of information on vaping products could help mitigate this potential social cost. |
||||||
Notes:
- 1. The time period for the analysis is 10 years from 2020–2029. The discount rate used for the analysis is 7%.
- 2. Numbers may not add up due to rounding.
Sensitivity analysis
A sensitivity analysis was conducted to show the estimated incremental costs resulting from the Regulations at a 3%, 7% and 10% discount rate (7% was used in this analysis). At a 3% discount rate, the total cost would be $8.3 million PV over 10 years, while a 10% discount rate would result in a $7.4 million PV incremental cost over the 10-year period. As indicated in the cost-benefit statement, the 7% discount rate results in a net cost of $7.7 million PV over 10 years.
Small business lens
It is estimated that approximately 75% of vape shops, 99% of manufacturers, 80% importers and 63% of G&C stores in the vaping industry are small businesses as per the Treasury Board of Canada Secretariat definition. footnote 19, footnote 20 G&C stores that are small businesses in the vaping industry will be impacted by the Regulations. G&C stores may see a reduction in sales as a result of fewer adults who smoke being exposed to vaping advertisements due to the restrictions on in-store advertising. It is anticipated that vape shops that rely primarily on sales at physical locations that are small businesses are less likely to be impacted by the Regulations given that the advertising and point of sale promotion restrictions do not apply as long as youth do not have access to their facilities. However, as vape shops generally use advertising such as brochures, websites and possibly social media, they may be impacted by the Regulations. Some manufacturers, importers and online retailers will also potentially be impacted, as they may see shifts in demand for their products and incur compliance costs following implementation of the Regulations.
It is anticipated that an estimated 1,879 small businesses will potentially incur incremental costs associated with the requirement of adding a health warning on advertisements of vaping products and the purchasing and installation of cabinets for vaping product storage. The small vape shops will incur incremental costs of updating indoor posters and adding health warning stickers to catalogues and brochures. Small online retailers will incur incremental costs of redesigning their websites. Small manufacturers and importers will incur incremental cost of updating posters and brochures that are sent to vape shops and G&C stores. Furthermore, some of the G&C stores in Alberta and Nunavut may incur costs of purchasing and installing cabinets for vaping product storage. The total cost is estimated at $3,567,608 PV over 10 years (or $507,947 annually). Thus, the incremental cost per impacted small business is estimated at $3,133 PV over 10 years (or $446 annually).
In developing the Regulations, approaches that balance the minimization of regulatory burden to business with the protection of youth from inducements to use vaping products were considered. For instance, the Regulations will still allow for promotional material on vaping products as long as they display the prescribed health warnings and are not accessible by minors. Such material will continue to aid many businesses in informing their customers on vaping products.
Flexible option
It is estimated that the Regulations will affect 1,879 small businesses, which are composed mostly of small manufacturers, vape shop owners and G&C store retailers.
Providing additional time for small businesses to comply with the Regulations was considered. However, a delayed implementation period for small businesses was deemed to be counter-effective in addressing the youth vaping problem. Therefore, the development of a flexible option was not performed.
Small business lens analysis — Impacts on all small businesses in the vaping industry
Number of small businesses impacted |
1,879 |
|---|---|
Number of analytical years |
10 |
Base year for costing |
2020 |
Compliance costs |
Annualized Value ($) |
Present |
|---|---|---|
Purchase and installation of closed cabinets (G&C stores) |
14,843 |
104,252 |
Update of indoor posters (vape shops that advertise their own products) |
37,673 |
264,600 |
Application of stickers on product catalogues (vape shops that advertise their own products) |
471 |
3,308 |
Application of stickers on product catalogues (vape shops that advertise products supplied by other manufacturers/importer) |
1,570 |
11,025 |
Production of brochures (manufacturers and importers) |
47,675 |
334,849 |
Production of indoor posters (manufacturers and importers) |
165,762 |
1,164,240 |
Redesign of websites (online retailers) |
239,954 |
1,685,333 |
Total |
507,947 |
3,567,608 |
Administrative costs |
||
None |
0 |
0 |
Total |
0 |
0 |
Total costs (all impacted small businesses) |
507,947 |
3,567,608 |
Cost per impacted small business |
446 |
3,133 |
Note: The table above refers to compliance cost impacts only. Small businesses will experience potential impacts on profits should the Regulations impact their sales.
One-for-one rule
There is no administrative burden on businesses as a result of the Regulations. Therefore, the one-for-one rule does not apply.
Regulatory cooperation and alignment
The following section describes any alignments with legislation in other jurisdictions that govern the types of promotion that are targeted by the Regulations.
Part 1 — Advertising and Point of Sale Promotion
Provincial legislation
Promotion at retail establishments is already restricted in all provinces and territories, except Alberta and Nunavut. British Columbia, Manitoba, Saskatchewan, Quebec, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, Yukon and the Northwest Territories prohibit the display and advertising of vaping products at retail establishments where youth have access and have requirements in place for the signs that show the availability and price of vaping products at retail establishments.
With regard to advertising outside the retail environment Saskatchewan, Manitoba, Prince Edward Island and the Northwest Territories restrict the advertising of vaping products on vehicles, buildings or outdoor structures such as billboards. Ontario prohibits advertising of vaping products in places of entertainment, such as cinemas and restaurants. The other jurisdictions (British Columbia, Alberta, New Brunswick, Nova Scotia, Newfoundland and Labrador, Yukon, Nunavut) have no measures in place to further restrict advertising in public places other than at retail establishments.
Quebec prohibits online sale of vaping products and only permits vaping product advertising in newspapers and magazines that have an adult readership of least 85%.
The Regulations further restrict vaping product advertising in Canada. They prohibit advertising where it can be seen or heard by young persons, including any public signage (billboards, bus shelter advertisements, etc.), on social media platforms, on TV, on radio and in all publications including those online, unless they are addressed and sent to an adult identified by name or only accessible to adults. The federal measures include restrictions on the promotion and display of vaping products at retail establishments that are accessible to youth in any province or territory that does not legislate in these areas. In particular, online advertising and point of sale promotions will be regulated across Canada under the Regulations.
International
Article 20(5) of the Tobacco Products Directive 2014/40/EU of the European Union requires member states to introduce restrictions on the advertising of e-cigarettes. While the Directive does not impose any rules on promotion or advertising of e-cigarettes that does not have “cross-border effects” and leaves regulation of such advertising to member states, the European Commission has implemented the same rules for cross-border advertising and promotion of e-cigarettes as the ones currently existing for tobacco products. These rules prohibit most cross-border promotional activity and include the following:
- Ban on press and publications that directly or indirectly promote e-cigarettes. There is an exception for trade publications and publications for outside of the European Union;
- Ban on radio promotions and audiovisual commercial communications; and
- Ban on direct or indirect promotion of e-cigarettes through “public or private contribution to any event, activity or individual person.”
Part 1 of the Regulations does not negatively impact the entry of new vaping products from other countries into the Canadian market. These importers will be limited in the same manner as new domestic vaping products manufacturers.
Part 2 — Required Information in Advertising (Health Warning)
Provincial legislation
All advertising across Canada are subject to the requirements of Part 2 of the Regulations, with the exception of advertising in provinces that require the display of a health warning on applicable advertisements.
Among the Canadian provinces, only Quebec requires a health warning on permitted vaping product advertisements in its jurisdiction. Online sale or advertising of vaping products is prohibited in Quebec. Hence, as per the exception set out in Part 2, the requirements to convey the health warning in vaping advertisements do not apply to Quebec, a province that already requires a health warning.
International
The United States requires the following health warning to be displayed on all vaping product advertisements: “WARNING: This product contains nicotine. Nicotine is an addictive chemical.” However, the Regulations only apply to promotions that originate in Canada. Hence, any promotion that originates from outside Canada, such as from the United States, is not subject to the Regulations. Section 31 of the TVPA sets out an exception regarding the sale of imported publications or the retransmission of radio and television broadcasts that originate outside Canada.
Nevertheless, the Regulations do not align with measures in the United States that are less restrictive for vaping promotions and that require a different health warning in advertisements of vaping products. The Regulations aim, among other things, to raise awareness of the harms of vaping products in general, not just those associated with nicotine. Therefore, the Regulations were designed in such a manner that the harms would be conveyed through the health warnings on advertisements of all vaping products, irrespective of whether or not the product contains nicotine.
Strategic environmental assessment
In accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals, a preliminary scan concluded that a strategic environmental assessment is not required.
Gender-based analysis plus (GBA+)
These Regulations are not expected to have any negative impacts on particular groups of Canadians on the basis of sex, gender, race, or ethnicity. However, it is likely that some population groups could be differentially impacted.
Sex differences relating to vaping
There are significant sex differences in vaping prevalence among youth aged 15 to 19 in Canada. In 2017, 8% of male youth and 4% of female youth had used an e-cigarette in the past 30 days. The same sex difference exists among Canadian students, with past-30-day use of e-cigarettes being higher among male (12%) than female (8%) students.
The 2019 public opinion research study Vapers Panel Survey to Measure Attitudes and Behaviours Regarding Vaping Products footnote 21 provides some insight on the attitudes and behaviours of Canadian regular vapers aged 15 years and older. Select findings regarding vaping information and advertising among regular vapers are the following:
- 28% of all participants recalled recent advertising or promotional materials about vaping.
- Recall was higher among youth (32%) and young adult vapers (37%) than among those aged 25 and older (24%).
- Participants were most likely to recall having seen information about particular brands of vaping devices.
- Social media was the top recalled source of advertising or promotional material.
The survey also revealed that male and female regular vapers were equally likely to recall having seen or heard vaping product advertising or promotional material in the past 30 days. Female regular vapers were more likely than male regular vapers to recall having encountered vaping product advertising or promotions through social media.
Other vulnerable population groups
It is generally observed that older adolescents/young adults and more educated groups are more likely to try a vaping product, which is a common pattern among early adopters of technologies. In Canada, 29% of young adults aged 20 to 24 had tried an e-cigarette in 2017 compared to 13% of adults aged 25 years and older.
CTS recognizes that certain groups of Canadians face smoking rates that are considerably higher than the general population and require more targeted action to ensure no one is left behind in Canada’s efforts to reach less than 5% of tobacco use by 2035. In particular, the prevalence of smoking among Indigenous peoples is approximately 2 to 5 times higher than among non-Indigenous Canadians; the smoking prevalence among LGBTQ+ persons is estimated to be in the ranges between 24% and 45% across different groups; and prevalence is higher in certain trades: for example, more than one third of construction workers smoked in 2011 (34%), followed by mining and oil and gas extraction workers (29%) and transportation and warehousing workers (29%). The evidence is clear that smoking is more prevalent in the lowest income groups and is a leading cause of health inequalities.
The uptake of vaping products by people who smoke in these vulnerable groups could have the potential to reduce health inequalities if they completely switch to vaping. However, there is limited data on vaping product use among these populations in Canada. Health Canada will continue to monitor the population and the health inequalities impacts of tobacco use. Efforts by Health Canada, the Public Health Agency of Canada and Indigenous Services Canada to reach these groups with higher rates of smoking through increased resources in tobacco programs will continue.
Implementation, compliance and enforcement, and service standards
Compliance promotion and outreach activities, including notices and the distribution of guidance material, aimed at informing manufacturers, importers, distributors and retailers of vaping products will be conducted in order to increase their level of awareness of the restrictions in these Regulations and to assist in achieving compliance.
The Government of Canada will actively monitor compliance throughout the supply chain, including manufacturers, importers, distributors and retailers. If federal inspectors have reasonable grounds to believe that the Regulations have been contravened, appropriate measures will be taken under the authorities of the TVPA, which could include warnings, compliance plans, seizures and even prosecution. Compliance and enforcement strategies will be consistent with the current overall approach to other prohibitions set out in the TVPA and its regulations. Compliance promotion activities will be followed by a progressive compliance monitoring and enforcement approach that will take into account any specific circumstances that may result from disruptions associated with the public health measures put in place to address the COVID-19 pandemic. The Government of Canada will provide additional guidance to the industry, as needed, on its compliance and enforcement approach in response to any disruptions associated with COVID-19.
The penalties for not complying with the Regulations are set out under Part VI of the TVPA. For the advertising restrictions, every person that contravenes the requirements is guilty of an offence and liable on summary conviction to a fine not exceeding $500,000 or to imprisonment for a term not exceeding two years, or to both (section 47 of TVPA). For the display of vaping products at retail, any person that contravenes the requirements is guilty of an offence and liable on summary conviction to a fine not exceeding $25,000 (section 48 of TVPA). For the requirement to display a health warning, every person that contravenes the requirements is guilty of an offence and liable on summary conviction to a fine not exceeding $50,000 or to imprisonment for a term not exceeding six months, or to both (section 44 of TVPA).
These Regulations do not relate to providing a service to the public or to industry; therefore, there are no service standards associated with these Regulations.
Contact
Mr. Mathew Cook
Manager
Scientific Regulations Division
Tobacco Products Regulatory Office
Tobacco Control Directorate
Controlled Substances and Cannabis Branch
Health Canada
Address locator 0301A
150 Tunney’s Pasture Driveway
Ottawa, Ontario
K1A 0K9
Fax: 613‑948‑8495
Email: hc.pregs.sc@canada.ca