Vaping Products Labelling and Packaging Regulations: SOR/2019-353
Canada Gazette, Part II, Volume 153, Number 26
Registration
SOR/2019-353 December 19, 2019
TOBACCO AND VAPING PRODUCTS ACT
CANADA CONSUMER PRODUCT SAFETY ACT
P.C. 2019-1416 December 18, 2019
Her Excellency the Governor General in Council, on the recommendation of the Minister of Health, makes the annexed Vaping Products Labelling and Packaging Regulations pursuant to
- (a) sections 17 footnote a and 33 footnote b of the Tobacco and Vaping Products Act footnote c; and
- (b) section 37 footnote d of the Canada Consumer Product Safety Act footnote e.
TABLE OF PROVISIONS
Vaping Products Labelling and Packaging Regulations
PART 1
Labelling — Awareness of Health Hazards from Use of Vaping Products
Interpretation
1 Definitions
Application
2 Retail sale — vaping products
3 Non-application
Purpose
4 Labelling — Tobacco and Vaping Products Act
Vaping Products Containing Nicotine
Nicotine Concentration Statement
5 Requirement — vaping product containing nicotine
6 Requirement — nicotine concentration
7 Placement — vaping device and vaping part
8 Placement — packaged vaping device and vaping part
9 Placement — refill vaping product
10 Placement — vaping products in kit
11 Placement — kit
12 Placement — prepackaged product
Health Warning
13 Requirement — vaping product containing nicotine
14 List of health warnings
15 Amended health warning
16 Placement — vaping device and vaping part
17 Placement — packaged vaping device and vaping part
18 Placement — refill vaping product
19 Placement — vaping products in kit
20 Placement — kit
21 Attribution
Vaping Products Without Nicotine
22 No nicotine
23 Permitted expressions
24 Placement
Presentation of Information
Official Languages
25 Required health warning and permitted expression
Technical Specifications
General
26 Integrity
27 Permanence
28 Legibility
29 Characters in text
30 Visibility
Nicotine Concentration Statement
31 Specific legibility rules
Health Warning
32 Text — health warning
33 Official languages — placement
34 Official languages — vaping product and package
35 Visibility
36 Specific legibility rules
Leaflet and Tag
37 Display of information
38 Specific legibility rule
39 Leaflet
40 Tag — visibility
41 Tag — safe handling
Attribution
42 Continuous text
PART 2
Labelling and Packaging — Protection of Human Health or Safety
Interpretation
43 Definitions
Application
44 Vaping products — consumer products
Purpose
45 Requirements — Canada Consumer Product Safety Act
Exception
46 Importation to bring into compliance or to export
Requirements
List of Ingredients
47 Contents
48 List of ingredients — placement
49 Maximum nicotine concentration
Child-Resistant Containers
50 Requirement — child-resistant container
51 Applicable standard
52 Maintain characteristics
53 Evaluation
54 Documents
55 Directions for opening and closing
56 Single-use immediate container
57 Toxicity information
58 Vaping device and vaping part
Presentation of Information
59 Languages, legibility and durability
60 Information in words — general rules
61 Order of presentation of ingredients
62 Directions to open and close
63 Hazard symbol — minimum diameter
64 Toxicity warning and first aid treatment statement
PART 3
Transitional Provision, Related Amendment and Coming into Force
Transitional Provision
65 Toxicity information
Related Amendment
Canada Consumer Product Safety Act
66 Consumer Chemicals and Containers Regulations, 2001
Coming into Force
67 July 1, 2020
SCHEDULE 1
SCHEDULE 2
Vaping Products Labelling and Packaging Regulations
PART 1
Labelling — Awareness of Health Hazards from Use of Vaping Products
Interpretation
Definitions
1 (1) The following definitions apply in this Part.
- display surface means the portion of the surface area of a vaping product or package on which the information referred to in this Part can be displayed. It does not include the surface area of the bottom, of any seam or of any concave or convex surface near the top or the bottom of a vaping product or package. (aire d’affichage)
- exterior package means a package that contains a vaping product and that is displayed or visible under normal or customary conditions of sale or use of the vaping product. (emballage extérieur)
- interior package means the innermost package of a vaping product, including a blister pack. (emballage intérieur)
- kit means a package that contains a collection of two or more units of vaping products. (trousse)
- main display panel means, in respect of a vaping product or package, the part of the display surface that is displayed or visible under normal or customary conditions of sale or use of the vaping product. It includes
- (a) in the case of a vaping product or package that has a rectangular cuboid shape, one of the largest sides of the display surface;
- (b) in the case of a vaping product or package that has a cylindrical shape, the larger of
- (i) the area of the top, or
- (ii) 40% of the area obtained by multiplying the circumference of the vaping product or package by the height of the display surface;
- (c) in the case of a bag, the largest side of the bag; and
- (d) in any other case, the largest surface of the vaping product or package that is not less than 40% of the display surface. (aire d’affichage principale)
- manufacturer does not include an individual or entity that only packages or labels vaping products on behalf of a manufacturer. (fabricant)
- vaping device has the meaning assigned by paragraphs (a) and (b) of the definition vaping product in section 2 of the Tobacco and Vaping Products Act. (dispositif de vapotage)
- vaping part has the meaning assigned by paragraph (c) of the definition vaping product in section 2 of the Tobacco and Vaping Products Act. (pièce de vapotage)
- vaping substance has the meaning assigned by paragraph (d) of the definition vaping product in section 2 of the Tobacco and Vaping Products Act. (substance de vapotage)
Interpretation — package
(2) In this Part, a reference to an exterior package or interior package does not include package liners or shipping containers or any outer wrapping, including a box, that is not displayed or visible under normal or customary conditions of sale or use of a vaping product.
Interpretation — refill vaping product
(3) In this Part, a reference to a vaping product that is intended to be used for the purpose of refilling another vaping product does not include a reference to a vaping device or vaping part.
Application of meanings in Tobacco and Vaping Products Act
(4) All other words and expressions used in this Part have the same meaning as in the Tobacco and Vaping Products Act.
Application
Retail sale — vaping products
2 (1) This Part applies to every vaping product that is intended for retail sale in Canada, as well as to its packaging.
Other means of furnishing — vaping products
(2) This Part also applies to every vaping product that is intended to be furnished by any means other than retail sale, as well as to its packaging.
Non-application
3 This Part does not apply to vaping products that are subject to the Food and Drugs Act.
Purpose
Labelling — Tobacco and Vaping Products Act
4 (1) For the purposes of sections 15.1 and 15.2 of the Tobacco and Vaping Products Act, this Part sets out the labelling requirements that manufacturers of vaping products must meet
- (a) in respect of information that is required to be displayed about vaping products and their emissions and about the health hazards and health effects arising from the use of those products and from their emissions; and
- (b) in respect of the manner of displaying the information referred to in paragraph (a) on vaping products and their packages, as well as on leaflets and on any tags attached to vaping products.
Permitted expressions
(2) For the purposes of sections 30.42 and 30.45 of the Tobacco and Vaping Products Act, this Part also sets out the requirements that manufacturers of vaping products must meet in respect of the manner of displaying certain non-mandatory expressions on vaping products and their packages.
Vaping Products Containing Nicotine
Nicotine Concentration Statement
Requirement — vaping product containing nicotine
5 Every vaping product that contains nicotine must display a nicotine concentration statement.
Requirement — nicotine concentration
6 The nicotine concentration statement must set out the concentration of nicotine in the vaping substance, expressed in milligrams per millilitre. The concentration must be preceded by “Nicotine — ” and followed by the unit of measure “mg/mL”.
Placement — vaping device and vaping part
7 The nicotine concentration statement must be displayed, in the case of a vaping device or vaping part that is not packaged, on the main display panel or on a tag.
Placement — packaged vaping device and vaping part
8 (1) The nicotine concentration statement must be displayed, in the case of a vaping device or vaping part that is packaged, on the main display panel of the exterior package.
Multiple layers of packaging
(2) If a vaping device or vaping part is packaged in multiple layers of packaging, the nicotine concentration statement must also be displayed on
- (a) the main display panel of the vaping device or vaping part, as the case may be; or
- (b) the main display panel of the interior package.
Placement — refill vaping product
9 (1) The nicotine concentration statement must be displayed, in the case of a vaping product that is intended to be used for the purpose of refilling another vaping product, on the main display panel of that refill vaping product.
Placement — packaged refill vaping product
(2) If the refill vaping product is packaged, the nicotine concentration statement must also be displayed on the main display panel of the exterior package.
Placement — vaping products in kit
10 (1) The nicotine concentration statement must be displayed, in the case of a vaping device or vaping part that is packaged in a kit, on
- (a) the main display panel of the vaping device or vaping part, as the case may be;
- (b) the main display panel of the interior package of the vaping device or vaping part, as the case may be; or
- (c) a tag.
Kit — refill vaping product
(2) The nicotine concentration statement must be displayed, in the case of a vaping product that is intended to be used for the purpose of refilling another vaping product and that is packaged in a kit, on the main display panel of that refill vaping product.
Placement — kit
11 (1) If vaping products that contain nicotine are packaged in a kit, the nicotine concentration statement that is required for each of those vaping products must be displayed on the main display panel of the kit.
One nicotine concentration statement
(2) However, if two or more of the vaping products in the kit have the same concentration of nicotine, the nicotine concentration statement that is applicable to those vaping products must be displayed only once on the main display panel of the kit.
Placement — prepackaged product
12 If a vaping product is a prepackaged product, as defined in subsection 2(1) of the Consumer Packaging and Labelling Act, and its identity is shown, as required under subparagraph 10(b)(ii) of that Act, in terms of its common or generic name or in terms of its function, the nicotine concentration statement must be displayed immediately below that name or function.
Health Warning
Requirement — vaping product containing nicotine
13 Every vaping product that contains nicotine must display a health warning.
List of health warnings
14 The health warning that is required for a vaping product is the health warning set out for that vaping product in the document entitled List of Health Warnings for Vaping Products, as amended from time to time and published by the Government of Canada on its website.
Amended health warning
15 Despite these Regulations, if a person has displayed, in accordance with this Part, a health warning that is set out in the List of Health Warnings for Vaping Products, as the list read immediately before the day on which the amended version of that list is published by the Government of Canada, the person may continue to so display that health warning during the period of 180 days after the day on which the amended list is published.
Placement — vaping device and vaping part
16 The health warning must be displayed, in the case of a vaping device or vaping part that is not packaged, on the main display panel or on a tag.
Placement — packaged vaping device and vaping part
17 (1) The health warning must be displayed, in the case of a vaping device or vaping part that is packaged, on the main display panel of the exterior package.
Exception — small exterior package
(2) However, if the main display panel of the exterior package has an area of less than 15 cm2, the health warning may be displayed elsewhere on the display surface of the exterior package or in a leaflet.
Multiple layers of packaging
(3) If a vaping device or vaping part is packaged in multiple layers of packaging, the health warning must also be displayed
- (a) if the main display panel of the interior package has an area of at least 15 cm2,
- (i) on the main display panel of the vaping device or vaping part, as the case may be, or
- (ii) on the main display panel of the interior package; and
- (b) if the main display panel of the interior package has an area of less than 15 cm2,
- (i) elsewhere on the display surface of the interior package, or
- (ii) in a leaflet.
Placement — refill vaping product
18 (1) The health warning must be displayed, in the case of a vaping product that is intended to be used for the purpose of refilling another vaping product, on the main display panel of that refill vaping product.
Exception — small refill vaping product
(2) However, if the main display panel of the refill vaping product has an area of less than 15 cm2, the health warning may be displayed on a tag.
Placement — packaged refill vaping product
(3) If the refill vaping product is packaged, the health warning must also be displayed on the main display panel of the exterior package.
Placement — vaping products in kit
19 (1) The health warning must be displayed, in the case of a vaping device or vaping part that is packaged in a kit, on
- (a) the main display panel of the vaping device or vaping part, as the case may be;
- (b) the main display panel of the interior package of the vaping device or vaping part, as the case may be; or
- (c) a tag.
Kit — refill vaping product
(2) The health warning must be displayed, in the case of a vaping product that is intended to be used for the purpose of refilling another vaping product and that is packaged in a kit, on
- (a) the main display panel of that refill vaping product; or
- (b) if the main display panel of the refill vaping product has an area of less than 15 cm2,
- (i) the main display panel of the interior package of a refill vaping product that is packaged, or
- (ii) a tag.
Placement — kit
20 (1) If a vaping product that contains nicotine is packaged in a kit, the health warning that is required for that vaping product must be displayed on the main display panel of the kit.
One health warning — kit
(2) However, if two or more of the vaping products in the kit contain nicotine, the required health warning need only be displayed once on the main display panel of the kit.
Attribution
21 If a manufacturer attributes a health warning, the manufacturer must do so by displaying the phrase “Health Canada” immediately beside or below the English version of the health warning and the phrase “Santé Canada” immediately beside or below the French version of the health warning.
Vaping Products Without Nicotine
No nicotine
22 A vaping product may display an expression permitted by section 23 if
- (a) in the case of a vaping substance, it does not contain nicotine; and
- (b) in the case of any other vaping product that contains a vaping substance, the product does not contain nicotine.
Permitted expressions
23 Only one of the following expressions is permitted to be displayed on a vaping product:
- (a) “Nicotine-free” in the English version of the expression and “Sans nicotine” in the French version of the expression;
- (b) “No nicotine” in the English version of the expression and “Aucune nicotine” in the French version of the expression; or
- (c) “Does not contain nicotine” in the English version of the expression and “Ne contient pas de nicotine” in the French version of the expression.
Placement
24 An expression permitted by section 23 must be displayed only on the display surface of a vaping product or of any package containing the vaping product.
Presentation of Information
Official Languages
Required health warning and permitted expression
25 The required health warning and any expression permitted by section 23 must be displayed in both official languages, in the same manner.
Technical Specifications
General
Integrity
26 (1) The customary method of opening a vaping product or package must not sever, otherwise damage or render illegible any letter, word or part of the required information or of an expression permitted by section 23.
Exception — blister pack
(2) Subsection (1) does not apply to an interior package that is a blister pack.
Permanence
27 Any required information and any expression permitted by section 23 must be irremovable.
Legibility
28 (1) Any required information must
- (a) be displayed in a standard sans-serif type that
- (i) is not compressed, expanded or decorative,
- (ii) is black on a white background, and
- (iii) as illustrated in Schedule 2, has a large x-height relative to the ascender or descender of the type; and
- (b) be clear and legible and remain so throughout the useful life of the vaping product, under normal or customary conditions of transportation, storage, sale and use.
Measurement of height of type
(2) The height of the type must be determined by measuring an upper case letter or a lower case letter that has an ascender or a descender, such as “b” or “p”.
Characters in text
29 Each character in the text of the required information must have the same font and type size.
Visibility
30 Any required information must not be concealed or obscured by any other information that is required or permitted to be displayed under the Tobacco and Vaping Products Act, any other Act of Parliament or any Act of the legislature of a province.
Nicotine Concentration Statement
Specific legibility rules
31 (1) A nicotine concentration statement that is displayed on a vaping product or package must be displayed
- (a) in a type that has a minimum height of 3 mm and a minimum body size of 8 points, if the main display panel has an area equal to or greater than 45 cm2;
- (b) in a type that has a minimum height of 2 mm and a minimum body size of 6 points, if the main display panel has an area equal to or greater than 10 cm2 but less than 45 cm2; and
- (c) in a type that has a minimum height of 1.5 mm and a minimum body size of 4.5 points, if the main display panel has an area of less than 10 cm2.
Measurement of height of type
(2) The height of the type must be determined in accordance with subsection 28(2).
Health Warning
Text — health warning
32 A health warning must be displayed in such a manner that
- (a) the first word is in upper case letters and bold type;
- (b) the remaining text is not in bold type and is in sentence case letters.
Official languages — placement
33 A health warning in one official language must be displayed immediately beside or below the health warning in the other official language and the two must not be combined.
Official languages — vaping product and package
34 In the case of a vaping product or package, the health warning in both official languages must be displayed on the same main display panel or display surface, as the case may be.
Visibility
35 A health warning that is displayed on a vaping product or package must
- (a) in the case of a vaping product or package that is cylindrical in shape, not extend beyond the main display panel;
- (b) in the case of a vaping product or package that has a rectangular cuboid shape, be displayed on the main display panel; and
- (c) in the case of a bag or any other package, be readable in its entirety under normal or customary conditions of sale or use of the vaping product, without further manipulation of the bag or package.
Specific legibility rules
36 (1) In the case of a health warning that is displayed on a vaping product or package, the health warning must be displayed
- (a) in a type that has a minimum height of 2 mm and a minimum body size of 6 points, if the main display panel has an area of less than 45 cm2; and
- (b) in a type that has a height and body size that results in the health warning in both official languages occupying not less than 35% of the main display panel, if the main display has an area equal to or greater than 45 cm2.
Measurement of height of type
(2) The height of the type must be determined in accordance with subsection 28(2).
Leaflet and Tag
Display of information
37 Any required information that is displayed in a leaflet or on a tag must be
- (a) positioned as close as possible to the top edge of the surface of the leaflet or tag, in such a manner that, if applicable, any nicotine concentration statement is immediately above the health warning; and
- (b) displayed in such a manner that it is readable in its entirety under normal or customary conditions of sale or use of the vaping product, without further manipulation of the leaflet or tag.
Specific legibility rule
38 (1) Any required information that is displayed in a leaflet or on a tag must be displayed in a type that has a minimum height of 2 mm and a minimum body size of 6 points.
Measurement of height of type
(2) The height of the type must be determined in accordance with subsection 28(2).
Leaflet
39 A leaflet that displays a health warning that is required for a vaping product must be inserted in the package containing the vaping product.
Tag — visibility
40 A tag must not conceal or obscure any required information that is displayed on the vaping product or its package under the Tobacco and Vaping Products Act, any other Act of Parliament or any Act of the legislature of a province.
Tag — safe handling
41 A tag must not interfere with the normal use or safe handling of the vaping product.
Attribution
Continuous text
42 (1) The attribution of a health warning must be displayed in such a manner that there is no intervening image or text between the attribution and the health warning.
Specific legibility rules
(2) The attribution of a health warning must be displayed in such a manner that
- (a) it meets the requirements set out in sections 28 and 29;
- (b) it is not in bold type; and
- (c) the height and body size of the type of the attribution are no larger than those of the type of the health warning.
Characters in text
(3) Each character in the text of the attribution must have the same font as the text of the health warning.
PART 2
Labelling and Packaging — Protection of Human Health or Safety
Interpretation
Definitions
43 (1) The following definitions apply in this Part.
- display surface means the portion of the surface area of an immediate container or of an exterior package on which the information referred to in this Part can be displayed. It does not include the surface area of the bottom, of any seam or of any concave or convex surface near the top or the bottom of the immediate container or exterior package. (aire d’affichage)
- exterior package means a package, other than an immediate container, that contains a vaping product and that is displayed or visible under normal or customary conditions of sale of the vaping product to a consumer. (emballage extérieur)
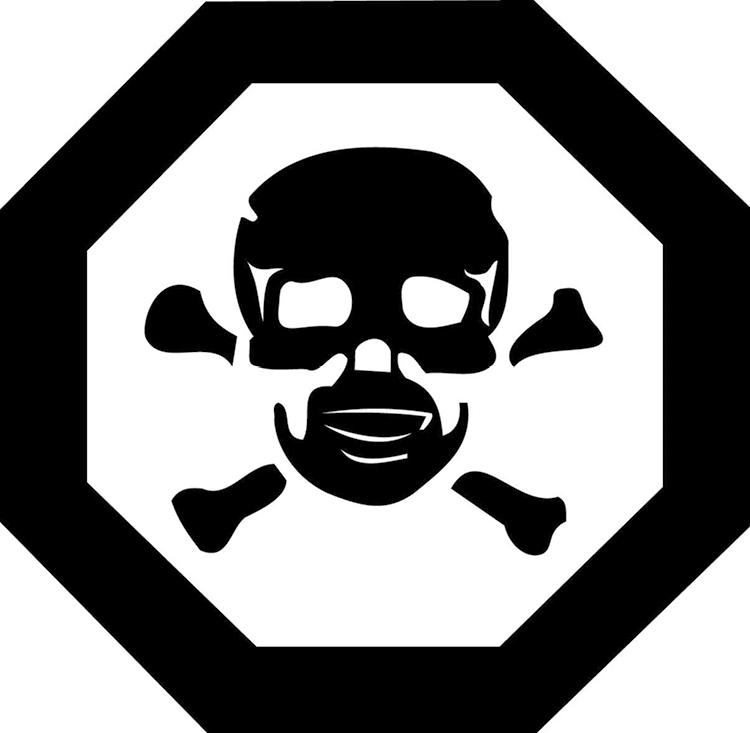
- hazard symbol means the pictograph and its frame as set out in Schedule 1. (pictogramme de danger)
- immediate container means the container, including a vaping device or vaping part, that is in direct contact with a vaping substance or which it is reasonably foreseeable will be in direct contact with such a substance. (contenant immédiat).
- main display panel means the part of the display surface that is displayed or visible under normal or customary conditions of sale to the consumer. It includes
- (a) in the case of a rectangular immediate container or exterior package, the largest side of the display surface;
- (b) in the case of a cylindrical immediate container or exterior package, the larger of
- (i) the area of the top, or
- (ii) 40% of the area obtained by multiplying the circumference of the immediate container or exterior package by the height of the display surface;
- (c) in the case of a bag, the largest side of the bag; and
- (d) in any other case, the largest surface of the immediate container or exterior package, as the case may be, that is not less than 40% of the display surface. (aire d’affichage principale)
- responsible person means
- (a) the manufacturer that, in Canada, manufactures a vaping device or vaping part or places a vaping substance in its immediate container; and
- (b) the importer, in the case of a vaping device or vaping part that is imported or a vaping substance that is imported in its immediate container. (responsable)
- vaping device has the meaning assigned by paragraphs (a) and (b) of the definition vaping product in section 2 of the Tobacco and Vaping Products Act. (dispositif de vapotage)
- vaping part has the meaning assigned by paragraph (c) of the definition vaping product in section 2 of the Tobacco and Vaping Products Act. (pièce de vapotage)
- vaping product has the same meaning as in section 2 of the Tobacco and Vaping Products Act. (produit de vapotage)
- vaping substance has the meaning assigned by paragraph (d) of the definition vaping product in section 2 of the Tobacco and Vaping Products Act. (substance de vapotage)
Interpretation of “should”
(2) If the word “should” is used in a standard that is referred to in this Part it is to be read as imperative, unless the context requires otherwise.
Application of meanings in Canada Consumer Product Safety Act
(3) All other words and expressions used in this Part have the same meaning as in the Canada Consumer Product Safety Act.
Application
Vaping products — consumer products
44 (1) This Part applies to vaping products that are consumer products.
Non-application
(2) For greater certainty, this Part does not apply to vaping products that are subject to the Food and Drugs Act.
Purpose
Requirements — Canada Consumer Product Safety Act
45 For the purposes of section 6 of the Canada Consumer Product Safety Act, this Part sets out requirements that must be met by vaping products that are consumer products.
Exception
Importation to bring into compliance or to export
46 (1) A person may import a vaping product that does not comply with a requirement of this Part for the purpose of
- (a) bringing the product into compliance with the requirement;
- (b) reselling the product to a manufacturer in Canada that will bring it into compliance with the requirement; or
- (c) exporting the product to another country.
Credible evidence
(2) A person that imports a vaping product for a purpose described in subsection (1) must, on the request of an inspector, provide credible evidence to the inspector that it is being brought into compliance or is being exported.
Requirements
List of Ingredients
Contents
47 (1) A list of ingredients is required for every vaping substance and must set out the common name, without abbreviation, of each ingredient that is present in the vaping substance. The list must begin with the title “Ingredients:” in the English version of the list and the title “Ingrédients :” in the French version of the list.
List of ingredients — “flavour”
(2) If any ingredients or combination of ingredients are added to a vaping substance solely to produce a particular flavour or combination of flavours, instead of being denoted in the list of ingredients by their common names, those ingredients must be denoted by the indication “flavour” in the English version of the list and “arôme” in the French version of the list.
List of ingredients — placement
48 (1) Subject to subsections (2) and (3), the list of ingredients must be displayed on the display surface of the immediate container of the vaping substance and on the display surface of any exterior package.
Exception — vaping device and vaping part
(2) If the immediate container is a vaping device or vaping part and it is not packaged, the list of ingredients must be displayed on a tag attached to the vaping device or vaping part, as the case may be.
Exception — size of main display panel
(3) If the main display panel of the immediate container has an area of less than 45 cm2, the list of ingredients must be displayed on the display surface of the exterior package or a tag attached to the immediate container.
Maximum nicotine concentration
49 A vaping product must not contain nicotine in a concentration of 66 mg/mL or more.
Child-Resistant Containers
Requirement — child-resistant container
50 (1) Every immediate container that is a vaping device or vaping part, as well as every other immediate container that contains a vaping substance that has a nicotine concentration of 0.1 mg/mL or more, must meet the requirements set out in section 51 for a child-resistant container.
Exception
(2) Subsection (1) does not apply if exposure to a vaping substance that is in a form other than an aerosol is impossible during the reasonably foreseeable use of the container.
Applicable standard
51 (1) A child-resistant container must
- (a) be constructed so that it can be opened only by operating, puncturing or removing one of its functional and necessary parts using a tool that is not supplied with the container; or
- (b) meet the child test protocol requirements of one of the following standards or a standard that is at least equivalent:
- (i) the Canadian Standards Association Standard CAN/CSA Z76.1-16, entitled Reclosable child-resistant packages, as amended from time to time,
- (ii) the International Organization for Standardization Standard ISO 8317:2015, entitled Child-resistant packaging — Requirements and testing procedures for reclosable packages, as amended from time to time, or
- (iii) the United States Code of Federal Regulations, Title 16: Commercial Practices, section 1700.20, entitled “Testing procedure for special packaging”, as amended from time to time.
Amended standard
(2) Despite paragraph (1)(b), if a person has applied the child test protocol requirements of one of the standards referred to in that paragraph to a child-resistant container, as the standard read immediately before the day on which an amended version of that standard is published, the person may continue to apply those requirements to the child-resistant container during the period of 180 days after the day on which the amended standard is published.
Maintain characteristics
52 A child-resistant container must maintain the characteristics of a child-resistant container throughout the useful life of the vaping substance that is placed in it, or in the case of a refillable container, throughout its useful life, under normal conditions of storage, sale and use.
Evaluation
53 (1) The responsible person, using good scientific practices, must evaluate
- (a) the compatibility of the vaping substance with its child-resistant container to determine that the chemical or physical properties of the substance will not compromise or interfere with the proper functioning of the container; and
- (b) the physical wear and stress factors and the force required for opening and closing the container to determine that the proper functioning of the container will be maintained for the number of openings and closings reasonably foreseeable for the size and contents of the container.
Definitions
(2) The following definitions apply in this section.
- good scientific practices means
- (a) for the development of test data, conditions and procedures that are in accordance with or equivalent to those set out in the Organisation for Economic Co-operation and Development document entitled OECD Guidelines for the Testing of Chemicals, as amended from time to time;
- (b) for laboratory practices, practices that are in accordance with the principles set out in the Organisation for Economic Co-operation and Development document entitled OECD Principles of Good Laboratory Practice, Number 1 of the OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, as amended from time to time; and
- (c) for human experience data, a peer-reviewed study of clinical cases. (bonnes pratiques scientifiques)
- human experience data means data, collected in accordance with good scientific practices, that demonstrates that injury to or poisoning of a human has or has not resulted from
- (a) exposure to a vaping substance; or
- (b) the reasonably foreseeable use of a vaping substance or immediate container by a consumer, including by a child. (données de l’expérience humaine)
Documents
54 (1) The responsible person must prepare and maintain documents containing the following information and must keep those documents for a period of at least three years after the day on which the child-resistant container is manufactured or imported:
- (a) the specifications critical to the child-resistant characteristics of the container, including
- (i) the physical measurements within which the container retains its child-resistant characteristics,
- (ii) if applicable, the torque that must be applied to open or close the container, and
- (iii) the compatibility of the container and its closure system with the vaping substance that is to be placed into it; and
- (b) the test results that demonstrate that the container and its closure system meet the requirements of a standard referred to in paragraph 51(1)(b).
Inspector’s request
(2) In the case of a vaping substance for which a child-resistant container is required, the responsible person must provide an inspector with any of the documents that the inspector requests within 15 days after the day on which the request is received.
Directions for opening and closing
55 (1) Directions for opening and, if applicable, closing a child-resistant container that meets the requirements of paragraph 51(1)(b) must be displayed on the closure system of the immediate container or on the display surface of the immediate container.
Exception — vaping device and vaping part
(2) However, in the case of an immediate container that is a vaping device or vaping part, the directions may be displayed
- (a) on a package containing the vaping device or vaping part, or on a leaflet that is inserted in the package; or
- (b) if it is not packaged, on a tag that is attached to the vaping device or vaping part.
Single-use immediate container
56 (1) Subject to subsections (3) and (5), the following statement must be displayed on the display surface of a child-resistant container that is an immediate container whose contents are to be used in their entirety immediately after the container is opened:
- “USE ENTIRE CONTENTS ON OPENING. THIS CONTAINER IS NOT CHILD-RESISTANT ONCE OPENED.”
- “UTILISER LA TOTALITÉ DU CONTENU APRÈS OUVERTURE. UNE FOIS OUVERT, LE CONTENANT N’EST PLUS UN CONTENANT PROTÈGE-ENFANTS.”
Exterior package
(2) Subject to subsection (4), the statement must also be displayed on the display surface of the exterior package, if any.
Exception — immediate container
(3) If the main display panel of the immediate container has an area of less than 45 cm2, the statement need not be displayed on the display surface of the immediate container.
Exception — exterior package
(4) If the main display panel of the exterior package has an area of less than 45 cm2, the statement must be displayed on a tag attached to the immediate container.
Exception — immediate container that is not packaged
(5) If the main display panel of the immediate container has an area of less than 45 cm2 and the immediate container is not packaged, the statement must be displayed on a tag attached to the immediate container.
Toxicity information
57 (1) The following information must be displayed on the display surface of the immediate container of a vaping substance that contains nicotine in a concentration of 0.1 mg/mL or more:
- (a) an exact reproduction, except with respect to size, of the hazard symbol set out in Schedule 1; and
- (b) the following toxicity warning and first aid treatment statement:
- “POISON: if swallowed, call a Poison Control Centre or doctor immediately.”
- “POISON : en cas d’ingestion, appeler immédiatement un centre antipoison ou un médecin.”
Exception — vaping device and vaping part
(2) If the immediate container is a vaping device or vaping part and it is not packaged, the information must be displayed on a tag attached to the vaping device or vaping part, as the case may be.
Exterior package
(3) The information must also be displayed on the display surface of the exterior package, if any.
Exception — exposure to vaping substance
(4) However, the information is not required if exposure to a vaping substance that is in a form other than an aerosol is impossible during the reasonably foreseeable use of the container.
Vaping device and vaping part
58 Despite sections 56 and 57, the information required by those sections need not be displayed on the display surface of an immediate container that is a vaping device or vaping part.
Presentation of Information
Languages, legibility and durability
59 The information required under this Part must
- (a) be displayed in both official languages;
- (b) be clear and legible and remain so throughout the useful life of the vaping substance, or in the case of a refillable immediate container, throughout its useful life, under normal conditions of transportation, storage, sale and use; and
- (c) be displayed in black type on a white background.
Information in words — general rules
60 (1) If the required information is set out in words, the words must be displayed in a standard sans-serif type that
- (a) is not compressed, expanded or decorative;
- (b) as illustrated in Schedule 2, has a large x-height relative to the ascender or descender of the type;
- (c) in the case of an immediate container or exterior package whose main display panel has an area equal to or greater than 45 cm2, has a minimum height of 3 mm and a minimum body size of 8 points; and
- (d) in the case of an immediate container or exterior package whose main display panel has an area of less than 45 cm2, has a minimum height of 2 mm and a minimum body size of 6 points.
Measurement of height of type
(2) The height of the type must be determined in accordance with subsection 28(2).
Characters in text
(3) Each character in the text of the required information must have the same font and type size.
Order of presentation of ingredients
61 (1) The ingredients set out in the list of ingredients referred to in section 47 must,
- (a) in the case of ingredients that are present in the vaping substance in a concentration of 1% or more, be set out in descending order of their proportions; and
- (b) in the case of ingredients that are present in the vaping substance in a concentration of less than 1%, be set out in any order and immediately following the ingredients referred to in paragraph (a).
Concentration of ingredient
(2) In this section, when the concentration of an ingredient in a vaping substance is expressed as a percentage, the percentage represents the ratio of the weight of that ingredient to the volume of the vaping substance.
Concentration — combination of flavours
(3) In the case of ingredients that are added to a vaping substance solely to produce a combination of flavours, the proportion of the total concentration of those ingredients must be used to determine the order of setting out the indication “flavour” in the list of ingredients.
Directions to open and close
62 (1) The directions for opening and, if applicable, closing a child-resistant container that are referred to in section 55 must be set out using either or both
- (a) words;
- (b) a diagram or self-explanatory symbol.
Official languages
(2) If the directions are set out in words, they may be displayed in one official language on the closure system of the child-resistant container and repeated elsewhere on the display surface of the container in the other official language.
Diagram or symbol — colour contrast
(3) If the directions are set out in a diagram or symbol, the contrast between the colour used for the information and that used for the background must be equivalent to at least a 70% screen of black on white.
Hazard symbol — minimum diameter
63 The hazard symbol referred to in paragraph 57(1)(a) must,
- (a) in the case of an immediate container or exterior package whose main display panel has an area equal to or greater than 45 cm2, have a diameter at least as large as the diameter of an imaginary circle that has an area equal to 3% of the main display panel; and
- (b) in the case of an immediate container or exterior package whose main display panel has an area of less than 45 cm2, have a diameter of at least 6 mm.
Toxicity warning and first aid treatment statement
64 The toxicity warning and first aid treatment statement referred to in paragraph 57(1)(b) must be displayed in such a manner that
- (a) the word “POISON” is in bold-faced, upper case letters; and
- (b) the statement is located immediately beside or below the hazard symbol referred to in paragraph 57(1)(a).
PART 3
Transitional Provision, Related Amendment and Coming into Force
Transitional Provision
Toxicity information
65 (1) The information on the toxicity of a vaping substance referred to in section 57 may, despite subsection 39(1) of the Consumer Chemicals and Containers Regulations, 2001, be displayed in accordance with these Regulations on a container that is an immediate container, other than a vaping device or vaping part, during the period beginning on the day on which these Regulations are published in the Canada Gazette, Part II, and ending on June 30, 2020.
Definition
(2) In this section, container has the same meaning as in subsection 1(1) of the Consumer Chemicals and Containers Regulations, 2001.
Related Amendment
Canada Consumer Product Safety Act
Consumer Chemicals and Containers Regulations, 2001
66 The Consumer Chemicals and Containers Regulations, 2001 footnote 1 are amended by adding the following after section 1:
Non-application
Vaping products
2 These Regulations do not apply to vaping products as defined in section 2 of the Tobacco and Vaping Products Act.
Coming into Force
July 1, 2020
67 (1) Subject to subsections (2) and (3), these Regulations come into force on July 1, 2020.
January 1, 2021
(2) Section 50 comes into force, in respect of an immediate container that is a vaping device or vaping part, on January 1, 2021.
Publication
(3) Section 65 comes into force on the day on which these Regulations are published in the Canada Gazette, Part II.
SCHEDULE 1
(Subsection 43(1) and paragraph 57(1)(a))
HAZARD SYMBOL
SCHEDULE 2
(Subparagraph 28(1)(a)(iii) and paragraph 60(1)(b))
ILLUSTRATION — STANDARD SANS-SERIF TYPE

REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Executive summary
Issues: Nicotine is the substance in tobacco products that causes dependence and drives the long-term use of these harmful products. There are concerns that the use of vaping products with nicotine by youth and non-users of tobacco products could lead to the use of tobacco products. Currently, there are no regulations that require the display of a warning about the addictiveness of nicotine or set out where and how information about the nicotine content of a vaping product be displayed on the product or its packaging.
Nicotine is highly toxic when ingested. Health Canada is aware of four fatalities of young children outside Canada and several non-fatal poisoning incidents in Canada related to the ingestion of vaping substances containing nicotine. Some requirements to help protect against vaping substance ingestion are already in place under the Canada Consumer Product Safety Act (CCPSA), but there are gaps in protection, particularly regarding refillable vaping devices and their parts.
Description: Health Canada is publishing a single set of regulations under the authorities of the Tobacco and Vaping Products Act (TVPA) and the CCPSA. The Regulations set out the requirements in two parts: labelling requirements pursuant to the TVPA, and labelling and child-resistant container requirements pursuant to the CCPSA. The labelling requirements include a list of ingredients and, for products containing nicotine, a health warning that nicotine is highly addictive, the concentration of nicotine, and warnings regarding the toxicity of nicotine when ingested. In addition, the Regulations set out expressions that may be used on the product or package to indicate when a vaping product is without nicotine. The Regulations also require refillable vaping products, including devices and their parts, to be child-resistant.
Cost-benefit statement: The Regulations impose costs on the vaping industry and the Government of Canada. A cost-benefit analysis estimated that the present value costs for industry related to the labelling provisions would be $3,234,500 (or an annualized average of $260,500), and those for the child-resistant container provisions would be $9,346,000 (or an annualized average of $753,000), which amount to a total of $12,580,500 (or an annualized average of $1,013,500). The government costs are estimated to be about $3,000,000 in present value (or an annualized average of $223,000) for compliance promotion, monitoring, and enforcement. The labelling requirements will result in benefits by increasing awareness of the health hazards of vaping products and will enable the people of Canada to make informed choices regarding their use, including avoiding exposure to nicotine. The combination of mandating toxicity warnings and child-resistant container requirements will also provide benefits by helping to protect against poisoning incidents and fatalities. The analysis suggested that the benefits of the child-resistant container provisions would exceed their costs if one emergency room visit was avoided every 7 to 29 days or one death was avoided every 24 to 92 years.
One-for-one rule and small business lens: The small business lens applies. The one-for-one rule also applies, as manufacturers and importers will have to obtain and maintain records with respect to the child-resistant container requirements. Nonetheless, having a single, product-specific regulation that sets out labelling requirements using authorities under the CCPSA and the TVPA will help to reduce the burden on small businesses.
Domestic and international coordination and cooperation: The Regulations will implement measures that are similar to those taken in the European Union for vaping products containing nicotine, which are required to display, among other things, a health warning regarding the addictiveness of nicotine and to provide information on the toxicity of nicotine. The child-resistant container requirements for refillable devices, and for stand-alone containers of vaping substances containing nicotine, are similar to the controls currently in place in the European Union. In the United States, a health warning regarding the addictiveness of nicotine is required for vaping products containing nicotine, and stand-alone containers of vaping substances containing nicotine must be child-resistant.
Issues
An Act to amend the Tobacco Act and the Non-smokers’ Health Act and to make consequential amendments to other Acts (referred to hereafter as “the Act”) received royal assent on May 23, 2018. The Act established a new legislative framework in Canada to govern vaping products, which consist of vaping substances, vaping devices and their parts. Vaping products are subject to the Tobacco and Vaping Products Act (TVPA) and either the Food and Drugs Act (FDA) or the Canada Consumer Product Safety Act (CCPSA), depending on whether or not the product is marketed for a therapeutic use. The Vaping Products Labelling and Packaging Regulations (hereafter “the Regulations”) are the first set of regulations under the Act to address the issues discussed below.
Nicotine toxicity
Nicotine is a highly toxic substance when ingested. A 2016 market study by Health Canada indicated that 86% of vaping substances marketed in Canada contain nicotine. Young children are curious by nature and explore their world with all their senses, including taste. footnote 2 To the knowledge of Health Canada, ingestion of vaping substances containing nicotine has resulted in four reported fatalities in young children. These incidents occurred in Israel in 2013, the United States in 2015, South Korea in 2016 and Australia in 2019. Additionally, Health Canada is aware of a number of non-fatal poisoning incidents, including some that occurred in Canada. The Canadian Hospitals Injury Reporting and Prevention Program data show that while there were no reports of injuries prior to 2013, there were 32 reported injuries related to vaping products between January 2013 and August 2018. footnote 3 Seventy-eight percent (78%) of the injuries reported were poisonings from ingesting a vaping substance, and 92% of those were among children aged four years or younger. Nearly two thirds (64%) of the vaping substances poisoning cases, among all age groups, specified that nicotine was involved. The rise in paediatric poisoning cases in Canada in recent years corresponds to a rise in sales of refillable vaping devices. footnote 4
Current requirements for vaping products under the Canada Consumer Product Safety Act
Upon royal assent of the Act, vaping products that contain nicotine and are not marketed for a therapeutic use were considered consumer products and subject to the CCPSA. These vaping products must comply with CCPSA provisions, which prohibit the manufacture, importation, advertisement or sale of consumer products that are a danger to human health or safety. Further, vaping substances containing nicotine at certain concentrations became subject to the Consumer Chemicals and Containers Regulations, 2001 (CCCR, 2001). These Regulations set out child-resistant container and toxicity warning requirements for stand-alone containers of vaping substances containing nicotine. The mandatory requirements imposed under the CCPSA and the CCCR, 2001 are intended to address risks posed by the acute toxicity of nicotine when ingested. Health Canada is currently enforcing these requirements. As described above, the existing requirements for vaping products regulated under the CCPSA allow Health Canada to address many of the risks posed by the acute toxicity of ingested nicotine.
However, there is one additional risk that was not addressed through the previous mandatory requirements. Vaping devices and their parts, including refillable tanks, did not need to meet the requirements from the CCCR, 2001 as a result of an exemption in the CCPSA that was put in place and came into force in May 2018. This exemption was put in place upon royal assent of the Act to allow industry time to source products that would meet the child-resistant container requirements.
Addictiveness of nicotine
Nicotine is a highly addictive substance. It is the substance in tobacco products that causes the addiction that drives long-term use and, as a result, repeated exposure to toxic chemicals in tobacco and its emissions. Vaping products deliver nicotine via inhalation of an aerosol formed when a nicotine-containing vaping substance is heated. In 2018, the United States National Academies of Sciences, Engineering and Medicine concluded that “there is substantial evidence that e-cigarette use results in symptoms of dependence on e-cigarettes.” footnote 5
Additional labelling concerns
A study conducted for Health Canada in 2017 showed diverse labelling practices among vaping product manufacturers. In particular, while the nicotine concentration did appear on most products, or their packaging, there was no consistency in how the information was presented. When present, there was also wide variation in the health warnings with few products indicating that nicotine is addictive. Given these additional findings and the concerns they raise, the powers of the TVPA are used to require that consumers be consistently informed about the presence of nicotine in a vaping product and about the addictiveness of nicotine. Also, specific expressions are prescribed in the Regulations to indicate that a vaping product is without nicotine.
Background
Tobacco use is the leading preventable cause of premature death and disease in Canada. Each year, approximately 45 000 people in Canada die from smoking-related diseases. Canada’s Tobacco Strategy aims to protect the health of the people of Canada, especially young people, from the dangers of tobacco use, including by helping the people of Canada quit smoking or reduce the harms of nicotine addiction.
While scientific knowledge is evolving, there is a consensus in the scientific community that for people who smoke, completely switching to vaping is less harmful than smoking conventional cigarettes. However, while vaping products may bring public health benefits if they reduce tobacco-related death and disease by helping adult tobacco users either quit or switch completely to a less harmful source of nicotine, these products are not harmless.
Most vaping products contain nicotine, which is highly addictive. Children and youth are especially susceptible to the risk of dependence.
Youth experimentation with and uptake of vaping could lead to tobacco use. Over a dozen studies show that among never-smoking youth, vaping significantly increases the risk of tobacco initiation.
Vaping products are also particularly harmful to youth and non-smokers because while they are less harmful than cigarettes, they emit chemicals during use that could negatively affect the health of youth and non-smokers. The potential short and long-term effects of vaping products remain unknown, and there is limited research on the effects of second-hand vapour.
Furthermore, nicotine is a highly toxic substance when ingested. Vaping products therefore pose health and safety risks, especially to young children who may gain access to the product and its contents in a household.
Vaping products were introduced commercially to the global marketplace in 2006. Over the past decade, Canada and the world have seen a rise in the popularity of vaping products. They are referred to by many different names, including e-cigarettes, electronic nicotine delivery systems, vapes, vape pens, etc., and vary in design and appearance. For the purposes of the Regulations, vaping products consist of vaping substances, vaping devices and their parts. The vaping substance, which includes substances commonly called e-liquids, is contained in a reservoir or tank within the device, and is normally drawn into an atomizer that creates an aerosol that is inhaled by the user. Technology continues to evolve rapidly in this area and there are many types of vaping device designs, such as
- closed devices that are not refillable;
- open devices that are refillable;
- devices that use cartridges that are prefilled and disposable;
- devices that use cartridges that are refillable; and
- devices that are completely customizable.
Vaping substances are primarily composed of propylene glycol and/or glycerol (sometimes referred to as vegetable glycerine), flavouring ingredients and often nicotine. A large number of vaping substances are available in Canada, with new formulations continually being introduced. Vaping substances may be sold in a stand-alone container ready-mixed by the manufacturer or custom-mixed in vaping shops. Vaping substances are also available in prefilled cartridge formats that are not intended to be refilled. Vaping substances are a recurring purchase, since they are consumed during vaping and are essential to the practice.
Legislative background
In May 2018, the Act amended the Tobacco Act to extend its scope to the manufacture, sale, labelling, and promotion of vaping products and to change its title to the Tobacco and Vaping Products Act (TVPA). Its purpose statement was also modified to include, among others, the goals of preventing the public from being deceived or misled with respect to the health hazards of using vaping products, and of enhancing public awareness of those hazards.
As defined in section 2 of the TVPA, vaping product means
- “(a) a device that produces emissions in the form of an aerosol and is intended to be brought to the mouth for inhalation of the aerosol;
- (b) a device that is designated to be a vaping product by the regulations;
- (c) a part that may be used with those devices; and
- (d) a substance or mixture of substances, whether or not it contains nicotine, that is intended for use with those devices to produce emissions.
- It does not include devices and substances or mixtures of substances that are excluded by the regulations, cannabis, as defined in subsection 2(1) of the Cannabis Act, cannabis accessories, as defined in that subsection, tobacco products or their accessories.”
Under the TVPA, the packaging, manufacture and sale of vaping products are prohibited unless the product and/or its package display, in the prescribed form and manner, the information required by regulations.
The Act also amended the CCPSA by replacing the previous wording of items 3 and 4 of Schedule 1 of that Act with the following:
- 3. Devices within the meaning of section 2 of the Food and Drugs Act, except a vaping product within the meaning of section 2 of the Tobacco and Vaping Products Act that is not subject to the Food and Drugs Act.
- 4. Drugs within the meaning of section 2 of the Food and Drugs Act, except a vaping product within the meaning of section 2 of the Tobacco and Vaping Products Act that is not subject to the Food and Drugs Act.
Pursuant to these amendments, Health Canada is authorized to administer the CCPSA for the purposes of addressing health or safety issues relating to vaping products including those containing nicotine, but not vaping products within the meaning of section 2 of the TVPA that are subject to the FDA.
Vaping products with or without nicotine that are marketed for a therapeutic use continue to be regulated under the FDA, while being regulated under the TVPA, except where expressly excluded by the Regulations Excluding Certain Vaping Products Regulated Under the Food and Drugs Act from the Application of the Tobacco and Vaping Products Act.
Interim application of the CCCR, 2001 under the CCPSA
At this time, Health Canada has identified nicotine to be the only known ingredient of concern in vaping substances, related to toxicity by ingestion. Health Canada has assessed the toxicity of nicotine when ingested and, on the basis of that assessment, has determined the following:
- 1. Vaping substances, which are to be sold as consumer products, containing equal to or more than 66 mg/mL nicotine footnote 6 meet the classification of “very toxic” under the CCCR, 2001 and are prohibited from manufacture, import, advertising or sale under section 38 of the CCCR, 2001.
- 2. Vaping substances, which are to be sold as consumer products, containing between 10 mg/mL and less than 66 mg/mL nicotine meet the classification of “toxic” and are subject to all applicable requirements under the CCCR, 2001 for toxic chemicals. Stand-alone containers of vaping substances intended for sale at retail are required to be sold in child-resistant containers, and to be labelled in accordance with the applicable CCCR, 2001 requirements, including a toxic hazard symbol on the container’s main display panel.
Applicability of sections 7 and 8 of the CCPSA
The CCCR, 2001 classification scheme for the determination of toxicity does not apply to ingredients present in concentrations of less than 1% (which in the Regulations is expressed as 10 mg/mL). Therefore, the CCCR, 2001 do not apply to vaping substances containing less than 10 mg/mL nicotine. However, a risk assessment conducted by Health Canada determined that nicotine at concentrations between 0.1 mg/mL and less than 10 mg/mL is potentially toxic when ingested and may contravene sections 7 and 8 of the CCPSA. As a consequence, in order to address this risk, Health Canada has communicated to industry that vaping substances within this range must adhere to all requirements of the CCCR, 2001 for “toxic” products, including the requirements for a child-resistant container.
The existing requirements and prohibitions will continue to apply to vaping substances that contain nicotine at concentrations of 0.1 mg/mL or more until the coming-into-force date of July 1, 2020. There are currently no child-resistant container requirements for refillable vaping devices or their parts and these specific provisions will come into force on January 1, 2021.
Recommendations regarding vaping products regulations
Canada is a Party to the World Health Organization Framework Convention on Tobacco Control (FCTC). Although vaping products are not captured in the scope of the FCTC, the World Health Organization (WHO) issued a report in 2016 on vaping products in response to a request made by the Conference of the Parties to the FCTC. footnote 7 Among other things, the report recommends that Parties that have not banned the importation, sale, and distribution of Electronic Nicotine Delivery Systems (ENDS) / Electronic Non-Nicotine Delivery Systems consider the following options to minimize health risks to users and non-users:
- Regulate appropriate labelling of devices and vaping substances;
- Require health warnings about potential health risks deriving from their use. Health warnings may additionally inform the public about the addictive nature of nicotine in ENDS; and
- Reduce the risk of accidental acute nicotine intoxication by requiring tamper evident / child-resistant packaging for vaping substances and leak-proof containers for devices and vaping substances, and limiting the nicotine concentration and total nicotine amount in devices and vaping substances.
Countries have adopted various regulatory strategies with respect to vaping products. According to a policy scan prepared by the Institute for Global Tobacco Control, there are 98 countries that have national/federal laws regulating vaping products, including laws related to the sale (including minimum age), advertising, promotion, sponsorship, packaging (child-resistant packaging, health warning labelling, and trademark), product regulation (nicotine volume/concentration, safety/hygiene, ingredients/flavours), reporting/notification, taxation, use (vape-free) and classification of vaping products. footnote 8 Of these countries, 28 have banned the sale of all types of vaping products and an additional 7 countries prohibit the sale of vaping products containing nicotine. footnote 9 In the countries that permit vaping products, 38 mandate the placement of health warnings on packaging and 31 have regulations on child-resistant packaging. footnote 10
In March 2015, Canada’s House of Commons Standing Committee on Health issued its report titled “Vaping: Towards a regulatory framework for e-cigarettes.” footnote 11 The Committee put forth 14 recommendations, one of which invited the Government of Canada to “… require that electronic cigarette components be sold in child-resistant packaging, and that all packaging clearly and accurately indicate the concentration of nicotine and contain appropriate safety warnings about the product.”
Objectives
The objectives of the Regulations are twofold, drawing from the authorities of the TVPA and the CCPSA.
The first objective of the Regulations is to use the authorities set out in the TVPA to help protect young persons and non-users of tobacco from exposure to, and dependence on, nicotine and to help prevent vaping product use leading to the use of tobacco products. More specifically, the Regulations aim to enhance awareness of the health hazards of using vaping products and prevent the public from being deceived or misled with respect to the health hazards posed by their use.
The second objective of the Regulations is to use the authorities set out in the CCPSA to help protect the health and safety of young children by reducing the risk that they ingest vaping substances containing toxic concentrations of nicotine. More specifically, the Regulations
- prohibit vaping products with nicotine concentrations of 66 mg/mL or more;
- require stand-alone containers of vaping substances containing nicotine in a concentration of 0.1 mg/mL or more to be child-resistant and display toxicity warnings on product labels;
- require refillable vaping devices and their parts to be child-resistant; and
- require all vaping substances to display an ingredient list on product labels.
Description
The Regulations set out requirements under the authority of the TVPA and the CCPSA. The provisions under the TVPA apply to vaping products and their packaging that are intended for retail sale or to be furnished footnote 12 by any means other than retail sale to a consumer in Canada. As mentioned in the “Legislative background” section, the TVPA applies to all vaping products, including those subject to the FDA, unless expressly excluded. The labelling requirements set out in Part 1 of the Regulations, which are under the authority of the TVPA, do not apply to those vaping products subject to the FDA. The requirements in Part 2 of the Regulations, which are under the authority of the CCPSA, apply only to vaping products that are consumer products, that is, those not subject to the FDA.
PART 1: Requirements under the authority of the Tobacco and Vaping Products Act
1. Vaping products containing nicotine
There are two labelling elements required for vaping products that contain nicotine: a nicotine concentration statement and a health warning about the addictiveness of nicotine. These labelling elements must be displayed on vaping products and/or their packaging, as the case may be, when the vaping product contains nicotine. In certain cases, the use of tags or leaflets is permitted. The information must respect the legibility and visibility requirements set out in the Regulations. The health warning must be displayed in both official languages.
For vaping substances in liquid form, Health Canada has developed a method, namely, Method C57.1: Determination of Nicotine at Low Concentration in Liquids used in Electronic Nicotine Devices by GC-MSD/FID, to assist regulated parties in determining if the substance contains nicotine. The title of this method is published on the Government of Canada website and the method is available upon request from the Government of Canada. footnote 13 For compliance and enforcement purposes, Health Canada may use the latest version of this method to determine when a vaping product is required to display the above noted labelling elements.
a) Nicotine concentration statement
For vaping products that contain nicotine, a statement of the nicotine concentration is required on the main display panel of the product or package. In certain cases, such as vaping devices and their parts that contain vaping substances with nicotine, this information may be displayed on a tag attached to the product. For the nicotine concentration statement, the word “Nicotine” is required and the concentration must be expressed in mg/mL for all forms of vaping substances.
b) Health warning
The nicotine addictiveness warning is “WARNING: Nicotine is highly addictive.” (“AVERTISSEMENT : La nicotine crée une forte dépendance.” in French). This health warning is required to appear on the main display panel of the product or package in both official languages. When a vaping product is sold without packaging or if the packaging or product is very small, this information may be displayed on a tag or in a leaflet, as the case may be.
The health warning is provided in a separate document entitled “List of Health Warnings for Vaping Products” and is available on the Government of Canada website. This document is incorporated by reference in the Regulations in a version that can be amended from time to time. This gives Health Canada flexibility to amend the health warning as new scientific evidence emerges on vaping products. Stakeholders will be notified of any proposed change to the list of health warnings. The Regulations allow for a 180-day transitional period after any change to the list to allow time to modify the health warnings that are used on the product and its packaging.
2. Vaping products that do not contain nicotine
The Regulations set out three permitted expressions that may be used when a vaping substance does not contain nicotine and, in the case of any other vaping product that contains a vaping substance, the product is without nicotine. These expressions are:
- Nicotine-free / Sans nicotine
- No nicotine / Aucune nicotine
- Does not contain nicotine / Ne contient pas de nicotine
The use of one of these expressions on the product or package is voluntary. It is the manufacturer’s responsibility to ensure that the permitted expression is used only on a product that does not contain any nicotine.
The purpose of these standardized expressions is to prevent the consumer from being deceived or misled with respect to the health hazards or effects of the product. The intent is to prescribe consistent language about the absence of nicotine, on the product or package, to allow the consumer to make an informed choice about their use of vaping products.
PART 2: Requirements under the authority of the Canada Consumer Product Safety Act
The Regulations maintain and extend the prohibition on the manufacture, importation, advertisement or sale of vaping products containing 66 mg/mL or more of nicotine, which was already in force under the CCCR, 2001. Since nicotine is the only ingredient that is acutely toxic by ingestion known to be present in vaping substances, the Regulations provide an opportunity to avoid using general toxicity classification criteria and instead set out the appropriate nicotine concentration considered to be very toxic.
The Regulations set out requirements for vaping substances containing nicotine in a concentration of 0.1 mg/mL or more to be packaged in a child-resistant container. Such containers are required to display a toxicity warning including the toxic hazard symbol. These requirements do not apply if the container does not permit exposure to the non-aerosolized form of nicotine under reasonably foreseeable use. The Regulations also set out requirements for refillable vaping devices and their parts to be child-resistant.
Each of the elements above is discussed specifically in the following sections.
Prohibitions
Health Canada used the literature value of 3.3 mg/kg for the LD50 of pure nicotine. The CCCR, 2001 has a prohibition on very toxic consumer chemical products, which are defined as having an LD50 of 50 mg/kg or less. Modelled on this CCCR, 2001 prohibition, the Regulations prohibit any vaping product containing nicotine at concentrations of 66 mg/mL or more from being manufactured, imported, advertised or sold.
Requirements
The Regulations set out a child-resistant container requirement for any vaping product that may hold a vaping substance containing nicotine in a concentration of 0.1 mg/mL or more. The child-resistant container requirements are modelled on those found in the CCCR, 2001. The child-resistant container requirement applies to refillable vaping devices and their parts, including component tanks or reservoirs that may hold vaping substances, and to stand-alone containers of vaping substances containing nicotine in a concentration of 0.1 mg/mL or more. The Regulations include a requirement for importers and manufacturers to obtain and maintain records to demonstrate that the container meets the child test protocol requirements in one of the prescribed acceptable standards. The person responsible for the vaping product is required to maintain documentation to demonstrate that the closure on the container maintains its function for the lifetime of expected use. This person is also required to include labelling, on the closure or display surface of the container, that instructs proper operation of the closure by the user. Finally, any documentation must be kept for a period of at least three years.
Any container of a vaping substance containing nicotine in a concentration of 0.1 mg/mL or more is required to have the toxic hazard symbol appear on the container and outer package, as applicable. A cautionary statement next to it is required to state, in both official languages: “POISON: if swallowed, call a Poison Control Centre or doctor immediately.” (“POISON : en cas d’ingestion, appeler immédiatement un centre antipoison ou un médecin,” in French). The hazard symbol and statement is designed to draw a person’s attention to the fact that nicotine is acutely poisonous if ingested, and to provide emergency instruction should poisoning occur.
All vaping substances, whether or not they contain nicotine, are required to display a list of ingredients to allow consumers to make informed choices regarding the products they choose to use.
Exceptions
The Regulations establish various exceptions to the requirements laid out above. The child-resistant container requirements and toxicity warning labelling are not required when exposure to the vaping substance is not reasonably foreseeable, such as when the product is not refillable. In addition, refillable vaping devices and their parts are not subject to labelling requirements for toxicity warning, unless they are sold prefilled with a vaping substance containing nicotine in a concentration of 0.1 mg/mL or more. In such cases, the toxicity warning is still required on the outer package, and where there is no outer package, the information is required on a tag attached to the product.
The Regulations set out an exception that persons responsible for the importation of a vaping product that does not comply with a requirement of the Regulations may import the product for the purposes of bringing it into compliance. This exception is considered necessary in order not to impede industry business models such as bringing foreign products into compliance with Canadian requirements for sale in Canada or export to another country.
Application of the CCCR, 2001
In order to clearly and efficiently regulate vaping products subject to the CCPSA under a new, product-specific regulatory instrument, the CCCR, 2001 has been amended.
Exclusion of vaping products from the application of the CCCR, 2001
In order for the labelling and child-resistant container requirements for vaping products to appear in only one regulation authorized under the CCPSA, the Regulations amend the CCCR, 2001 to remove vaping products from the application of the CCCR, 2001. Even though these products are removed from the application of the CCCR, 2001, they continue to be subject to the CCPSA.
An order to fix the date for the repeal of subsection 4(4) of the CCPSA
An order to fix the date for the repeal of subsection 4(4) of the CCPSA (which excludes vaping devices and their parts from the application of the provisions of the CCCR, 2001) has been published at the same time as the Regulations. This subsection of the CCPSA was an interim measure intended to be in effect until product-specific regulations were published under the CCPSA. Since the Regulations remove the applicability of the CCCR, 2001 to vaping products, it is appropriate to repeal subsection 4(4) of the CCPSA at this time.
Coming into force
The requirements of the Regulations come into force on July 1, 2020, including the requirements for stand-alone containers of vaping substance containing nicotine to be child-resistant. There is one exception. The coming-into-force date related exclusively to the child-resistant container requirements for refillable vaping devices or their parts is January 1, 2021.
The interim application of the CCCR, 2001 continues for vaping substances containing nicotine subject to the CCPSA until July 1, 2020.
Until July 1, 2020, a stand-alone container of a vaping substance containing nicotine may meet the toxicity warning requirements in the Regulations or those in the CCCR, 2001.
Regulatory development
Consultation
In August 2017, Health Canada published a consultation paper on potential regulatory measures that were under consideration for vaping products. footnote 14 Ten potential measures for the regulation of vaping products were set out, four of which relate to the objectives of the Regulations. These were
- that all vaping products that contain nicotine display their nicotine concentration in milligrams/millilitre (mg/mL);
- that any vaping product be considered to contain nicotine if nicotine is present at a concentration of 0.1 mg/mL or higher;
- that vaping products that contain nicotine display a warning such as “WARNING: This product contains nicotine. Nicotine is an addictive substance. Use of nicotine during pregnancy may harm the fetus.”; and
- that products that contain vaping substances display a complete list of ingredients in descending order by weight.
In response to this consultation, a total of 105 comments were received from academics; the public; other levels of government; industry, including the health products, vaping and tobacco industries; non-governmental organizations; public health groups; and retailers, including vape shops. Support was strong for all four labelling measures.
With regard to the health warnings, some stakeholders felt that certain aspects should be similar to tobacco labelling. These suggestions included that the warnings be graphic in nature, be rotated, occupy a minimum percentage of the package and that the packaging be plain in colour and design. In addition, some stakeholders indicated that the warnings for vaping products must be balanced with other messaging, such as relative risk statements, in order to support harm reduction and that the warnings must stay current with the science.
The industry expressed concerns that some smaller businesses would need sufficient time to deplete stock of existing labels. In addition, requiring that all flavouring ingredients be identified in the list of ingredients would be difficult for the industry, and it was suggested that the use of the term “flavour” in the list of ingredients would make it easier for industry. Concerns were also raised about the methodology and its ability to detect nicotine at a concentration of 0.1 mg/mL. Industry suggested that a minimum of 0.5 mg/mL should be considered for the amount of nicotine to require the health warnings and that the amount of nicotine should be displayed either in percent or mg/mL. In contrast, some public health groups and municipal/provincial/territorial governments commented that a vaping product should be considered to contain nicotine at any detectable level, as opposed to the proposed 0.1 mg/mL.
A summary of the comments received is available on the Government of Canada website. footnote 15
In response to comments about the need to stay current with the science, the Regulations incorporate by reference the List of Health Warnings for Vaping Products in a version that can be amended from time to time. This will allow Health Canada to be flexible and responsive to emerging science and technology for modifying these health warnings. When a change to the List of Health Warnings for Vaping Products is made, a transitional period of 180 days will be allowed for industry to comply with the change. Since there is only one health warning required at this time, there is no need for rotation of health warnings. To accommodate the concern that vaping products and their packaging can often be very small in size, the Regulations permit the use of tags and leaflets in certain cases.
In response to industry’s concerns about the listing of all flavour ingredients, the term “flavour” (“arôme” in French) must be indicated in the list of ingredients rather than the common name for the flavouring ingredients. In addition, in response to concerns raised about the availability of laboratory methods to determine the amount of nicotine at or below 0.1 mg/mL, a test method, Method C57.1: Determination of Nicotine at Low Concentration in Liquids used in Electronic Nicotine Devices by GC-MSD/FID, has been developed by Health Canada to determine nicotine at low concentrations. The title of this method is published on the Government of Canada’s website, and the method is available upon request from the Government of Canada. footnote 16
In response to concerns about a sufficient transition time to implement the requirements, the coming-into-force date for most provisions has been set to July 1, 2020, to provide industry with a transitional period of at least 180 days from registration to deplete its stock of existing products and labels.
The statement “Use of nicotine during pregnancy may harm the fetus” is not included in the Regulations due to feedback received from scientific experts indicating that there are limitations in the current scientific knowledge to support its inclusion for vaping products at this time.
The consultation document provided general information about the application of the CCPSA and the CCCR, 2001 to vaping products. The document indicated that the CCCR, 2001 would apply to all vaping products containing between 10 mg/mL and less than 66 mg/mL of nicotine. While concentrations of nicotine between 10 mg/mL and less than 66 mg/mL would be captured by the CCCR, 2001, a risk assessment of the toxicity of nicotine when ingested provides support for the position that vaping substances containing between 0.1 mg/mL and less than 10 mg/mL of nicotine that lack suitable toxicity labelling and child-resistant containers may constitute a danger to human health or safety for the purposes of sections 7 and 8 of the CCPSA. The consultation document also signalled that the packaging and labelling requirements would apply to all vaping products that do, or that may, contain nicotine, including refillable vaping devices.
In addition, a Notice to Industry was posted online and mailed to stakeholders in October 2017. This notice outlined, in greater detail, the regulatory requirements of the CCCR, 2001 for child-resistant containers and toxicity labelling for vaping products that would be effective upon royal assent of the Act.
While there was strong support for child-resistant containers to be required for vaping substances containing nicotine, there was strong opposition to requiring vaping devices to meet child-resistant container requirements. Industry stakeholders stated that virtually all vaping devices are imported from China, that there were very few vaping device models available that would meet the child-resistant container requirements, and that manufacturers would be unwilling to develop compliant models for the small Canadian market. Several industry stakeholders indicated that requiring vaping devices to meet child-resistant container requirements would have a devastating effect on the Canadian vaping industry. As a result, the Act was amended by Parliament to exempt from the CCCR, 2001, as an interim measure, devices and parts as defined in paragraphs (a) to (c) of the definition of a vaping product in section 2 of the TVPA.
Comments received were supportive of toxicity labelling on vaping substances, although some stakeholders expressed general concern that limited label space may make compliance with the CCPSA and other government labelling requirements problematic.
Health Canada posted the Guidance on Vaping Products Not Marketed for a Therapeutic Use on May 23, 2018, and notified industry members of its availability. footnote 17 This document signalled Health Canada’s intention to introduce specific regulations for vaping products under the CCPSA. Industry stakeholders were also provided the opportunity to take part in a webinar on this issue and were able to seek further clarification of the proposal during the webinar’s question and answer session. Health Canada inspectors continue to be available to respond to questions from industry.
Public opinion research
Health Canada conducted public opinion research (POR) on the labelling elements that are set out in the Regulations. The research was conducted in two phases and the participants included adults who smoke and adults who vape.
The findings from phase 1, the exploratory stage, showed a large gap related to vaping product information among both adults who vape and those who smoke, in particular the lack of knowledge about the health effects and health hazards of using vaping products. Participants who use vaping products, as well as participants who smoke but do not vape, expressed a clear interest in learning more about vaping.
In phase 2, labels showing the labelling elements considered under the authority of both the TVPA and CCPSA were tested on various vaping product and packaging configurations. The labelling elements tested included a nicotine concentration statement, a message on the addictiveness of nicotine, the CCCR, 2001 toxic hazard symbol shown with and without the word “POISON,” a first-aid treatment statement and a list of ingredients. In addition, variations in the wording of the health warning on nicotine addictiveness and the expressions that would indicate that the product does not contain nicotine were tested.
Participants indicated that they were interested in mandatory labelling information that would include an ingredient list, the nicotine content, and warnings or information related to health hazards. However, as presented in the mock-ups used in the POR, the information conveyed was not fully understood and some labelling elements were interpreted in multiple ways. Participants were often unable to clearly identify and differentiate the risks conveyed by the various labelling elements, which resulted in the meaning of each individual statement or component being weakened. In addition, some of the labelling elements were considered too vague or incomplete to provide effective guidance to consumers.
In response to the findings of the POR testing, modifications to the placement and wording of certain labelling elements of the Regulations were made:
- The warning reads, in both official languages: “POISON: if swallowed, call a Poison Control Centre or doctor immediately” (“POISON : en cas d’ingestion, appeler immédiatement un centre antipoison ou un médecin” in French).
- The statement above works by combining the specific hazard statement of “POISON” with the first aid treatment statement to better clarify the risk posed by the nicotine in the vaping substance. That is, ingestion of the nicotine in the vaping substance is acutely toxic. This warning must be placed alongside the hazard symbol anywhere on the display surface, which is different, in terms of the location of the warning, from the requirements in the CCCR, 2001, whereby it must be located on the main display panel.
- The nicotine concentration statement is co-located with the addictiveness warning, in all cases except those where the label is very small. Placing these two messages together provides the consumer with the complete messaging (i.e. this product contains nicotine and nicotine is addictive) while limiting the amount of information that must be repeated on a small label.
Prepublication in the Canada Gazette, Part I
The proposed Vaping Products Labelling and Packaging Regulations were published on June 22, 2019, in the Canada Gazette, Part I, for a 75-day consultation period that ended on September 5, 2019.
A number of mechanisms were used to inform stakeholders of the publication and to invite them to submit comments on the proposal:
- On June 21, 2019, Health Canada issued a bilingual press release announcing the publication of the proposed Regulations and soliciting feedback on the proposal.
- On June 21, 2019, a link was added to the Government of Canada’s “Health-related consultations” web page, in English and French, with a brief description of the proposed Regulations, a link to the publication and a request for comments.
- On June 21, 2019, a link was added to the Government of Canada’s “Vaping product regulation” web page in English and French.
- On June 21, 2019, a link was added to the Government of Canada’s “Consulting with Canadians” web page in English and French.
- On June 21, 2019, a link was added to the Government of Canada’s “Consumer products and cosmetics” web page in English and French.
- On June 21, 2019, direct bilingual email notifications providing a link to the publication were sent to
- approximately 14 975 subscribers of Health Canada’s Consumer Product Safety newsletter;
- approximately 1 980 subscribers of the CCPSA newsletter; and
- approximately 400 vaping business establishments identified through market surveys.
- On June 21, 2019, a bilingual message was sent by email to over 600 stakeholders (industry associations, industry members, public health stakeholders and consumers). The message informed recipients of the proposed Regulations and provided instructions for submitting comments. Recipients were encouraged to forward the email to other interested parties.
- On June 27, 2019, Global Affairs Canada issued a notification, in English and French, of the proposed Regulations according to the standard procedures for notification of the World Trade Organization Technical Barriers to Trade Committee.
A total of 124 responses were received including 14 from the general public; 22 from non-governmental organizations (NGOs); 62 from public health organizations and healthcare providers; 12 from other levels of government including school boards; 2 from academics and researchers; and 12 from industry including manufacturers, importers, distributors, retailers and associations. All comments were reviewed and taken into consideration when finalizing the Regulations.
Comments received from the general public, NGOs, public health organizations, healthcare providers, other levels of government and academics voiced general support for the proposed measures. They believe that labelling and packaging measures will enhance awareness of the health hazards of using vaping products and prevent the public from being misled or deceived with respect to product addictiveness. They also believe that the measures will protect young children by reducing the risk of accidental ingestion of toxic concentrations of nicotine. Many of these respondents requested additional measures to further protect the public, especially youth, from harms associated with vaping. Most other respondents, including industry and retail respondents and some members of the general public, also supported the overall intent of the Regulations, but felt that they may not achieve the objectives or that some measures would be costly and challenging to implement.
Health Canada has noted all comments received during the consultation. Many of the same comments were expressed during the consultation in 2017 and were carefully considered during the development of the Regulations. The remaining comments received were of a technical nature and Health Canada’s responses are described below. Some respondents also provided suggestions that were considered to be outside the scope of the Regulations and many of these are summarized below.
Health warning size and placement
Some respondents including members of the general public, NGOs, academics, public health organizations and other levels of governments requested that health warnings be similar to the large and prominent warnings required on packages of cigarettes and little cigars. Several commenters suggested health warnings take up at least 75% of the package space and many suggested that the health warning occupy a minimum surface area. Others requested that health warnings be required to be positioned on the top of packages for maximum visibility and be displayed on two sides of the same package.
Several vaping industry respondents requested adjustments to health warning sizing such as allowing smaller warnings and flexibility to change warnings on smaller packages (i.e. displaying French and English messages separately). Others requested requirements to include health warnings on either the package or the product itself, but not both.
Response: The information submitted during the consultation was carefully reviewed by Health Canada and did not change the outcome of Health Canada’s assessment on health warning size or placement on vaping product packaging. Health Canada considered the size of the health warning necessary to be prominent and the available space to display all required information and has determined that the regulatory requirements do not need to be modified.
Health warning content and rotation
NGOs, public health organizations and healthcare providers, academics, and most submissions from the general public were supportive of the health warning on nicotine addictiveness. One respondent requested the addition of a mandatory statement stating that “This product contains nicotine.” Many also requested including additional health warnings about vaping and heart disease, stroke, lung disease, cancer, pregnancy and risks particular to youth.
Some respondents requested the addition of relative risk statements encouraging smokers to completely switch from smoking tobacco to vaping products. Several respondents requested that information about smoking and vaping cessation be included on packaging. This could include quitline service phone numbers and websites which are currently displayed on packages of cigarettes and little cigars.
Some respondents suggested the addition of symbols and graphics to make the health warnings more noticeable. One public health authority requested culturally appropriate messaging that does not reinforce stigma or stereotypes associated with other substances.
Some respondents requested an additional mandatory statement that vaping products are not intended for youth. In contrast, one public health organization recommended that health warnings should not directly state that the product should not be used by youth.
Many respondents were supportive of requiring health warnings in a document that is accessible online and that can be modified by Health Canada without a regulatory amendment. Some respondents requested that a requirement be added for multiple health warnings to rotate across packages. One respondent requested the entire content of the warning (e.g. colour, font size and use of images and symbols) be included in the document to allow Health Canada to make changes to the entire content of the health warning without a regulatory amendment.
Response: Health Canada has carefully considered the comments but has not modified the regulatory requirements. Health Canada will continue to monitor the emerging evidence around the health effects and health hazards associated with vaping and has the flexibility to modify the List of Health Warnings for Vaping Products document as needed. There is currently one health warning, but should additional warnings be warranted, regulatory requirements around message rotation could be proposed. Note that compliance with the requirement regarding the display of the prescribed health warning on nicotine addictiveness does not exempt manufacturers or retailers from any other obligation at law to warn consumers of the health hazards arising from the use of their products.
Legibility
Some vaping industry respondents requested reduced font sizes for displaying health warning and nicotine concentration information on small packages. They indicated that fitting all of the required information on smaller packages would be challenging. In contrast, some public health organizations and NGOs requested larger font sizes for nicotine concentration statements, stating that the minimum font of 2 mm or 6 points is too small to be clear and noticeable. There were also requests to harmonize font sizes for consistency between the different regulatory requirements including health warnings, nicotine concentration statements, list of ingredients and toxicity information (i.e. requirements of parts 1 and 2 of the Regulations).
Several industry respondents requested that the legibility requirements for expressions permitted on products without nicotine be removed, since these are optional statements.
Two industry respondents requested permission to display text that contrasts with the background colour rather than prescribing black type on a white background. They noted that the requirement could be costly to implement on blister packs with silver foil lining and that the purpose of the Regulations is not to standardize the appearance of vaping products or their packaging.
Response: Health Canada has modified the regulatory requirements to align font height and point size across parts 1 and 2 for the required information. Health Canada has removed most legibility requirements for permitted expressions on products without nicotine. However, the requirement to preserve the permitted expressions’ integrity when opening the package has been maintained.
Health Canada has not modified the regulatory requirements to permit variances to black type on white background. Due to the acute toxicity of nicotine and the relatively small package sizes involved with vaping products, it is important that consumers be readily able to find safety-related information in an emergency situation. Black type on a white background clearly separates health and safety-related information from other information. It also maintains optimal legibility and noticeability for all required information.
Defining vaping products with and without nicotine
Many industry respondents commented on the different requirements for vaping products that contain nicotine versus vaping products without nicotine. Two respondents requested more precise definitions in Part 1 of the Regulations. Some respondents requested that the nicotine concentration of 0.1 mg/mL be used to define whether or not a vaping product is considered to contain nicotine, since it is the threshold set out in Part 2 of the Regulations for requirements for child-resistant containers and toxicity warning labelling for certain vaping products.
Health Canada also received technical comments about Health Canada’s Method C57.1: Determination of Nicotine at Low Concentration in Liquids used in Electronic Nicotine Devices by GC-MSD/FID (e.g. repeatability and reproducibility). One vaping product manufacturer indicated the need for a method with a lower limit of quantification. Another vaping product manufacturer requested that the method in ISO 20714:2019 entitled E-Liquid – Determination of nicotine, propylene glycol and glycerol in liquids used in electronic nicotine delivery devices – Gas chromatographic be used instead of Health Canada’s Method C57.1.
Response: Health Canada has removed the incorporation by reference of Method C57.1. Regulated parties can still choose to use this method to determine if a vaping product contains nicotine. Method C57.1 has been validated to determine the presence of nicotine at low concentrations in vaping liquids (lower than ISO method 20714:2019) and it may be used by Health Canada for compliance monitoring and enforcement purposes. The title of the method is published on the Government of Canada’s website, and the method is available upon request from the Government of Canada. footnote 18
Manufacturers of vaping products that contain nicotine must ensure that products display specific information as set out in the Regulations, including a health warning and nicotine concentration statement.
Manufacturers of vaping products may choose to promote their products that do not contain nicotine. If they choose to promote such products, they can display one of the permitted expressions in the Regulations on their products or packaging. Vaping product manufacturers are responsible for providing information about their vaping products that is accurate and not false or misleading.
Nicotine concentration statement
Many respondents agreed with the proposal to standardize the nicotine concentration statement and express the information in mg/mL. One respondent requested that the nicotine concentration be required on the device itself, while another asked if devices sold without prefilled pods would be required to display the mandatory information.
Some respondents indicated that Health Canada should mandate a method and tolerance level for the statement. A few respondents were under the impression that Method C57.1, referenced in the proposal, was the method prescribed for measuring the nicotine concentration. Several respondents specifically requested the use of the ISO 20714:2019 E-liquid – Determination of nicotine, propylene glycol and glycerol in liquids used in electronic nicotine delivery devices – Gas chromatographic method. Other respondents commented that Health Canada should oversee the testing of products to ensure that the labelled concentration is correct.
Response: The Regulations set out the requirement for a statement of the nicotine concentration of vaping products that contain nicotine to provide consumers with information about the presence of nicotine in vaping products. A nicotine concentration statement is not required on vaping products such as devices or parts that do not contain a substance with nicotine when they are sold or otherwise furnished.
Health Canada does not prescribe a method or tolerance level for measuring the nicotine concentration of vaping products. Vaping product manufacturers are responsible for providing accurate information in the nicotine concentration statement and can use analytical methods of their choice to measure and validate concentrations.
Maximum nicotine concentration
Most public health organizations, healthcare professionals, academics and researchers, NGOs and some respondents from the general public requested that the maximum nicotine concentration for vaping products be lowered from 66 mg/mL because of risks associated with nicotine beyond acute poisoning. Many respondents requested a limit of 20 mg/mL to align with limits set in several other jurisdictions (including the European Union, Israel, Iceland and South Korea) and indicated that this would reduce the risk of youth and non-smokers becoming addicted to nicotine. Some respondents also indicated this would mitigate specific risks to adolescents related to nicotine exposure and developing brains. One NGO also indicated that a limit of 20 mg/mL in vaping products would mitigate negative cardiovascular and respiratory impacts on youth and adults. Some public health organizations indicated that the 20 mg/mL maximum nicotine concentration would further protect against ingestion poisoning incidents and fatalities, poisoning from regular use, accidental overconsumption and self-harm.
In addition to comments around reducing the maximum nicotine concentration limit for vaping products, many respondents were concerned with the impact of vaping products made from nicotine salts and some respondents requested lower maximum nicotine concentration levels for nicotine salt products. Others requested a full prohibition of nicotine salts for non-therapeutic purposes to protect youth and non-smokers. Some respondents also requested the introduction of limits to the amount of nicotine allowed in closed capsules and new requirements around device features such as cartridge sizes and maximum battery voltage requirements. Such requirements would be intended to limit the amount of nicotine available to users of vaping products.
Response: Health Canada has carefully considered the comments but has not modified the regulatory requirements to further restrict the maximum allowable nicotine concentration in vaping products.
Health Canada will continue to monitor emerging evidence around vaping and may consider additional actions to limit nicotine concentration in vaping products in the future for reasons other than their ingestion toxicity.
The maximal concentration limit for nicotine of 66 mg/mL is based on a peer-reviewed toxicity evaluation of the ingestion of pure nicotine. The limit is consistent with how a very toxic consumer chemical is identified in the CCCR, 2001. footnote 19
Expressions indicating vaping products do not contain nicotine
Some public health organizations and members of the general public requested that displaying an expression indicating that a vaping product does not contain nicotine be mandatory on the packaging of all such products. They also requested that a health warning be required on these packages to help inform consumers that such products are not risk-free. One NGO requested permitting only a single expression indicating a vaping product does not contain nicotine to avoid the potential for consumers to interpret different meanings for different expressions.
Response: Health Canada has not modified the regulatory requirements. Vaping product manufacturers that choose to indicate on their products or packaging that they do not contain nicotine have the option to display one of the three permitted expressions. The Regulations do not require vaping product manufacturers to indicate that their products do not contain nicotine, only that they use one of the permitted expressions when they choose to make this marketing claim.
Ingredient labelling and flavour
Some vaping industry respondents requested clarity on the term “flavour” as it appears in the required ingredient list. They commented that the requirement as written could capture many ingredients that add a flavour but are not flavouring agents. For example, they indicated that propylene glycol, glycerol and nicotine all have a flavour that can contribute to the overall flavour of the vaping substance, but these ingredients are not flavouring agents. Other comments from public health organizations indicated that without further clarity on the meaning of the term, some ingredients could be hidden from users if they were captured by the general term “flavour” in the ingredient list. These stakeholders stated that, as it was proposed, a manufacturer could argue that such an ingredient does add flavour, even though it is not an ingredient used solely for the purpose of adding flavour.
Response: Health Canada has modified the requirement to clarify the meaning of the term “flavour” as capturing any ingredient that is added to a vaping substance solely to produce a particular flavour.
Immediate container
One vaping industry respondent requested clarification on the defined term “immediate container,” which references the concept of the immediate container being one in which a vaping substance is directly placed. These respondents appear to have interpreted this to mean that a vaping device could be an immediate container in the case where vaping parts (i.e. cartridges or pods that hold or may hold a vaping substance) are directly placed into the device. Further, based on a misunderstanding of the definition, an industry respondent suggested that it should explicitly exclude vaping devices that interface with a vaping part in which a vaping substance is directly placed, as this would exclude devices sold without any vaping substance from the requirement to be labelled with an ingredient list.
Response: Health Canada has modified the defined term “immediate container” to clarify that it is the container with which a vaping substance is in direct contact or with which it is reasonably foreseeable that such a substance will be in direct contact.
Note that immediate containers that do not contain a vaping substance do not require an ingredient list.
Child-resistant container requirements
Both public health respondents and industry respondents provided support for the child-resistant container requirements for stand-alone containers of vaping substances containing nicotine used to refill vaping devices or vaping parts. However, some vaping industry respondents indicated that these requirements for containers that are refillable vaping devices should be removed from the Regulations for reasons set out in the following paragraph.
Two industry respondents indicated their disagreement with the supplemental cost analysis conclusion that child-resistant devices are currently available on the global marketplace. They also indicated that a reduction in consumer choice would be seen, should these requirements be implemented. More comments from industry respondents disputed the existing requirements for child-resistant devices contained within the European Union’s Tobacco Products Directive, Directive 2014/40/EU (TPD). footnote 20 These respondents also disputed the statement that the requirements in the European Union have translated to the global availability of devices that are already child resistant. Other industry respondents highlighted that there would be many technical challenges to overcome in order to implement child resistance for refillable vaping devices. Some industry respondents indicated that a lack of specific testing methodologies for vaping device tanks or atomizers would present challenges. Additional comments from industry respondents questioned the number of poisoning incidents associated with nicotine containing vaping devices as compared to those associated with stand-alone containers of vaping substances containing nicotine.
In contrast, several public health respondents indicated that all vaping devices and parts, even those that are prefilled cartridge formats that are not intended to be refilled, should be packaged in child-resistant containers. These respondents also indicated that the child-resistant container requirements should be applied whether or not a vaping substance contains nicotine.
Response: Health Canada has carefully considered all of the comments received and has maintained the child-resistant container requirements for refillable vaping devices and parts. Health Canada has been transparent with its intention to set out child-resistant container requirements for refillable vaping devices and parts since 2017. There is an abundance of information, as described below, suggesting that the Regulations are not likely to require the redesign of child-resistant refillable vaping devices or parts, because such products are likely to be already readily available. However, in response to the concerns noted by two industry respondents, Health Canada has adjusted the quantitative costs for industry, in Table 1 within the “Benefits and costs” section of this document, to reflect the possibility that there may be redesign costs for refillable vaping devices or their parts. Similarly, Health Canada has adjusted the quantified but not monetized negative impacts to industry in Table 1 to reflect the possibility that some vape shops may experience a profit loss if they do not identify and stock compliant vaping devices or their parts before the child-resistant requirements for refillable vaping devices and their parts come into force.
Health Canada is confident that child-resistant refillable vaping devices and their parts are readily available today on the global marketplace, and that laboratories capable of conducting the child test protocols referenced in the Regulations are numerous in North America and in other global markets.
Through discussions with the European Union and certain Member State regulators (the United Kingdom and Denmark), Health Canada has confirmed that the European Union TPD’s child-resistant container requirements apply to refillable devices and their parts, as well as stand-alone containers of vaping substances containing nicotine. According to publicly available information, the TPD was published in 2014 and Member States were provided a two-year period to implement legislation reflecting the Directive. For example, in the United Kingdom, the Tobacco and Related Products Regulations 2016 which implement the TPD were made on April 18, 2016, and they came into force a month later on May 20, 2016. Retailers had until 20 May 2017 to sell through stock of products that did not comply with the labelling and product composition requirements of the TPD. This means that manufacturers and importers of products to the European market are already supplying child-resistant refillable vaping devices in order to comply with the legislation in the Member States. For example, in the United Kingdom, a producer is anyone who manufactures or imports vaping products or who re-brands any product as their own, and retailers do not have any specific requirements unless they also qualify as a producer. The Regulations are aligned with this approach, where it is the manufacturer or importer in Canada who is responsible for obtaining and maintaining the documents regarding the compliance of their products with the child-resistant container requirements.
Health Canada’s 2019 supplementary cost-analysis report, described in the “Benefits and costs” section of this document, also supports the conclusion that child-resistant refillable vaping devices are available on the global marketplace. This report states that “The availability of child-resistant devices in Europe and a potential shift in consumer preferences to closed devices suggest that the child-resistant container requirement that Health Canada is considering would pose relatively minor direct compliance costs for Canadian importers of open vaping devices.” In October 2019, Health Canada conducted an additional online market search, and identified a number of overseas manufacturers that supply a wide variety of refillable vaping devices and their parts that are promoted as child-resistant. Over 150 models of vaping devices or their parts were identified with documentation to support that their opening mechanisms are consistent with child-resistant designs, for example those requiring two independent opening actions (i.e. push down and turn) and/or that they have declared their product to be child and tamper-proof. These designs are likely to meet the child-resistant container requirements set out in the Regulations. Many of the identified models were available in a range of tank volumes. It was found that most of the identified products are marketed under the same popular brands that are already carried by Canadian importers, distributors and retailers, and would be recognized by adults who vape in Canada. The wide variety of refillable vaping devices identified as child-resistant indicates that consumers should not be faced with reduced product choice.
As part of the work to confirm the availability of child-resistant refillable vaping devices, a large North American testing laboratory for conducting child test protocols told Health Canada that they have been contracted to test vaping devices. Over the past two to three years, they have seen various designs for making the refillable vaping devices or refillable vaping parts child-resistant, and have observed that the designs have improved over time. It is noted that there are several laboratories that operate in North America and other global markets that can conduct the child test protocols on vaping devices and their parts specified in the Regulations.
The Regulations provide two pathways that can be used to comply with the child-resistant container requirements. One is to utilize an inherent design of the vaping device or part where a tool is required to open the container. Another is to utilize a container that meets the child test protocol requirements of one of three identified industry standards, or another standard that is at least equivalent. The child test protocols referenced by the Regulations are general testing procedures. Any immediate container with a closure can be tested according to one of the three child test protocols and there is no need to develop a new procedure for specific tank or atomizer designs.
The Regulations require containers of refillable vaping products, including devices and their parts, to meet the child-resistant container requirements. Nicotine is a highly toxic substance when ingested. Exposure to nicotine is possible when refillable vaping devices or their parts are not child-resistant and they are filled with a vaping substance containing nicotine. Young children mimic adult behaviour and it is reasonably foreseeable that a vaping device in a home could be picked up, manipulated and mouthed by a young child. It is consistent with the CCCR, 2001 to require containers of toxic chemicals to be child-resistant.
The child-resistant container requirements have not been extended to all vaping devices and their parts. Health Canada considers that vaping devices and their parts that are not intended to be refilled present a lower likelihood of access to a vaping substance when used as designed. Health Canada will continue to monitor the emerging evidence around ingestion toxicity risks for vaping substances containing nicotine. Risk management measures may be considered in the future if further health or safety protections are warranted.
The child-resistant container requirements have not been modified to apply to vaping products that contain nicotine in a concentration of less than 0.1 mg/mL. Nicotine is the only ingredient that is acutely toxic by ingestion known to be present in vaping substances. As stated above, this approach is consistent with the CCCR, 2001 to require containers of toxic chemicals to be child-resistant.
Toxicity information and hazard symbol
A number of public health organizations and some healthcare providers suggested extending the toxicity information labelling requirements across all vaping products, including closed pod vaping products, disposable products and non-refillable products. It was recommended to have the toxicity warnings on products as an injury prevention measure for situations that may be encountered such as product leakage, puncture, tampering or post-production modification.
Some respondents recommended an alternative hazard symbol such as the “acute toxicity” pictogram used by the Workplace Hazardous Materials Information System (WHMIS) or a new standardized nicotine warning symbol. One industry respondent recommended that toxicity labelling on 60 mL containers of vaping substances containing nicotine should align with labelling requirements of the CCCR, 2001, which specifies labelling with the specific hazard statement, positive and negative instructions, and the complete first aid treatment statement on the container. One public health organization recommended including a child-safety alert message to warn consumers about the potential risk of leaving products where children may have access to them.
One public health organization recommended placing a health hazard pictogram on a vaping substance that does not contain nicotine. This was suggested to warn about the potential health effects on pregnant women, children and infants from inhalation or ingestion of propylene glycol, and to warn about the possible allergic reactions and other health effects from exposure to vegetable glycerine.
Response: Health Canada has carefully considered the comments, but has not modified the requirements for the toxicity warning and first aid treatment statement. As described under the toxicity warning provisions, the immediate container must be labelled with the required information if the user may be exposed to the non-aerosolized form of the vaping substance containing nicotine during reasonably foreseeable use.
The hazard symbol in the Regulations aligns with the toxic hazard symbol required by the CCCR, 2001, which is intended to alert the public about the poisoning risk of toxic consumer chemical products. Over the years, the public has become familiar with the hazard symbols that appear on consumer chemical products, and Health Canada has been active in educating about their meaning and precautions to be taken for products labelled with the symbols. The toxic hazard symbol is important as a consistent reminder for parents and caregivers to securely close a container and to keep it stored out of sight and reach of children. Health Canada encourages parents, caregivers and educators to teach children at an early age that the hazard symbol means “Danger. Don’t touch it.” The WHMIS symbol is not suitable for use on a consumer product, as it is intended to alert and provide safety information on hazardous products in the workplace. Introducing a new standardized nicotine warning symbol may lead to confusion and may result in potential injury from product mishandling.
In terms of aligning completely with the labelling requirements for other consumer products containing hazardous substances, the Regulations use a modified approach because of the additional Part 1 labelling requirements. The toxic hazard symbol, toxicity warning and first aid treatment statement in the Regulations are considered sufficient to alert consumers and provide information on the acute toxicity associated with the ingestion of nicotine containing vaping substances. The Regulations require the warning and first aid treatment statement to be placed alongside the hazard symbol, which is expected to enhance consumers’ understanding of the danger and encourage them to take reasonable steps to safely handle and store the products.
With regard to the recommendation for a health hazard pictogram on vaping substances that do not contain nicotine, there is insufficient evidence at this time to suggest that propylene glycol, vegetable glycerine or other common ingredients would pose an acute ingestion toxicity concern when used in vaping products. In contrast, nicotine at concentrations commonly found in vaping substances is known to be acutely toxic. In order to maintain consistency with Health Canada’s approach to other consumer chemical products, only vaping products that contain nicotine in a concentration of 0.1 mg/mL or more are required to display the toxic hazard symbol.
Health Canada will continue to monitor emerging evidence around ingestion toxicity associated with vaping substances and may consider future risk management measures if further health or safety protections are warranted.
Coming into force
A number of vaping industry respondents commented that an implementation period of 180 days would be too short and that more time would be required to comply with all requirements, especially with respect to the new child-resistant container requirements for refillable vaping devices and their parts. In addition to requesting a separate extension on the implementation period for child-resistant container requirements, the respondents requested an extended implementation timeline for wholesalers and retailers, to allow them to sell through non-compliant inventory.
Some respondents recommended implementing the Regulations as quickly as possible to reduce health and safety risks to Canadians without delay.
Response: Health Canada has carefully considered the comments and has modified the coming-into-force timelines for the child-resistant container requirements for refillable vaping devices and their parts. Health Canada understands that the time required for a testing laboratory to complete a child test protocol on a product ranges from approximately three to six weeks. Health Canada is aware of several laboratories that operate in North America and other global markets that conduct at least one of the child test protocols specified in the Regulations. In order to provide additional time for industry to source and test refillable vaping devices or their parts for child resistance, an additional 180 days have been provided. That is, a January 1, 2021, coming-into-force date is specifically set out for refillable vaping devices and their parts related exclusively to the child-resistant container requirements.
All other regulatory requirements will come into force on July 1, 2020. This provides a transition period for industry of at least 180 days from registration of the Regulations, while requiring the measures to be implemented quickly to maximize their public health and safety benefits.
Out of scope
Many submissions provided recommendations for vaping products that were out of scope for this initiative. These included requests to
- amend the definitions of vaping products in the TVPA;
- establish a registration regime for new vaping devices and products introduced to the Canadian market;
- establish a licensing regime for vendors of vaping products;
- restrict online sales of vaping products;
- establish a new taxation scheme for vaping products;
- establish the same packaging and labelling measures for cannabis vaping products;
- restrict promotion of vaping products;
- introduce plain and standardized appearance requirements for vaping product packaging;
- limit the physical characteristics of devices;
- consider measures to prevent post-production modification, puncturing, tampering and leakage of vaping products;
- restrict vaping product flavours;
- revise Health Canada’s position on harm reduction and introduce relative risk statements between vaping and tobacco use;
- update Canada’s Tobacco Strategy to address vaping;
- enhance public education activities related to vaping in partnership with and by providing funding to other organizations;
- establish a national centralized point of contact for poison control and require that product labels provide the national telephone number;
- complete additional research around vaping including its health impacts and effectiveness for smoking cessation; and
- consult further with industry.
Response: These recommendations fall outside of the scope of the Regulations, but are being carefully examined by Health Canada, along with similar comments that were received from the consultation from April to May 2019 “Reducing Youth Access and Appeal of Vaping Products: Potential Regulatory Measures.” footnote 21
These Regulations are one measure to help minimize the risk associated with vaping for the people of Canada with respect to both the exposure to and dependence on nicotine as well as acute poisoning from nicotine. They are among the suite of actions related to vaping currently being pursued by Health Canada including a national public education campaign to increase awareness about the health harms and risks associated with vaping products and additional compliance and enforcement activities. Health Canada is continuing to explore other regulatory and non-regulatory measures to help protect youth and non-users of tobacco products from exposure to and dependence on nicotine.
Modern treaty obligations and Indigenous engagement and consultation
All people of Canada, including Indigenous peoples, will benefit from the public health and product safety approach taken in these Regulations. Requirements for child-resistant containers and toxicity warning labelling will help to protect young children from nicotine poisoning. The nicotine concentration statement and the health warning will provide information on the health hazards of using vaping products containing nicotine to enhance public awareness and prevent the public from being deceived or misled with respect to these hazards.
Instrument choice
Option 1: Status quo
Under this option, Health Canada would have continued to rely on the CCCR, 2001 for vaping substances containing nicotine in concentrations of 10 mg/mL or more. Further, sections 7 and 8 of the CCPSA would still have been relied upon for vaping substances containing nicotine in concentrations between 0.1 mg/mL and less than 10 mg/mL, since these concentrations are potentially toxic when ingested but are not addressed by the CCCR, 2001. Some regulated parties may have found this approach to be overly complex and unclear. In addition, there are some features of the CCCR, 2001 that limit their suitability to address the ingestion toxicity risk posed by vaping substances containing nicotine, including the following:
- 1. Given that subsection 4(4) of the CCPSA excluded vaping devices and their parts from the scope of the CCCR, 2001, the authorities of the CCCR, 2001 could not be applied to require refillable tanks of open vaping devices and their parts to be child resistant.
- 2. The CCCR, 2001 prescribe a criteria-based system that addresses the inherent hazards associated with a consumer chemical product. Before publication of the Regulations, the entirety of the CCCR, 2001 applied to vaping substances, which meant that each responsible person was required to evaluate their substances against hazard categories and exposure routes that were not likely applicable, such as corrosivity. This presented an unnecessary burden on industry, given that Health Canada has identified nicotine to be the only known ingredient of concern in vaping substances related to toxicity by ingestion.
- 3. Nicotine is potentially toxic when ingested at concentrations below 10 mg/mL; however, the authorities of the CCCR, 2001 were not applicable in cases where a toxic ingredient was present in a concentration below that limit.
- 4. The POR completed in 2018 revealed that some elements of the CCCR, 2001 toxicity warning were confusing to people who use vaping products. In some cases, they interpreted the warning to mean that inhaling the aerosol was poisonous, rather than the intended message that ingesting the liquid was poisonous.
Products that pose a danger to human health or safety under the Canada Consumer Product Safety Act
On the basis of a risk assessment conducted by the Department, Health Canada determined that vaping substances containing nicotine in a concentration of 0.1 mg/mL or more may pose a danger to human health or safety and initiated compliance and enforcement action. However, because the danger to human health or safety provisions of the CCPSA are of general application to consumer products and do not refer specifically to vaping products, some industry members may not have been aware of the extent of their obligation to ensure that containers of vaping substances containing nicotine in a concentration of 0.1 mg/mL or more had effective protections against ingestion risks.
Industry practice in Canada
In Canada, at the time of prepublication in the Canada Gazette, Part I, the Electronic Cigarette Trade Association recommended that their members voluntarily display the nicotine concentration and a list of ingredients on the product label. A 2017 study conducted for Health Canada showed diverse labelling practices among manufacturers. While information about the amount of nicotine did appear on most products, there was no consistency in how that information was presented. The absence of regulations specific to this type of information could have led to consumers being deceived or misled with respect to the health hazards of using vaping products.
For the reasons outlined above, Option 1 was not the preferred option.
Option 2: Introduction of Vaping Products Labelling and Packaging Regulations under the TVPA and the CCPSA
The second option involved making new regulations, jointly made under the TVPA and the CCPSA. It would set out requirements for vaping products that address the acute toxicity of nicotine when ingested, and include child-resistant container and labelling requirements for toxicity, modelled after those in the CCCR, 2001 of the CCPSA. It would also include mandatory labelling requirements that support the purposes of the TVPA.
This is the option represented by the Regulations. It was chosen for the following reasons:
- 1. Relying on the application of the provisions of the CCPSA, for vaping substances containing nicotine in concentrations between 0.1 mg/mL and less than 10 mg/mL, and relying on the CCCR, 2001, for vaping substances containing nicotine in concentrations of 10 mg/mL or more, to address the nicotine ingestion risk was likely to cause confusion for industry. Regulations that set out specific regulatory requirements enable greater clarity, certainty and predictability for industry, as well as for Health Canada officials engaged in compliance and enforcement activities.
- 2. Exposure to nicotine is possible when refillable vaping devices or their parts are not child resistant. These products did not need to meet the requirements of the CCCR, 2001 through an exemption in the CCPSA that came into force in May 2018. Child-resistant devices are now available on the global marketplace. Regulations that set out requirements for child-resistant containers for refillable vaping devices and their parts would render the exemption redundant.
- 3. For vaping products that are regulated under both the TVPA and the CCPSA, there were no labelling requirements to inform consumers regarding the addictiveness of nicotine, to display the nicotine concentration in a standardized format or to provide a list of ingredients. The CCCR, 2001 only required a product label to disclose hazardous ingredients that contribute to the classification conclusion and that are present at a concentration of 1% or more.
- 4. Single, product-specific regulations for vaping products enable Health Canada to more readily address future health or safety risks captured by the scope of the CCPSA, such as other toxicological, mechanical or electrical risks related to vaping products, through amendments to the Regulations. This also benefits industry by having all CCPSA requirements for vaping products under one regulation.
- 5. Single, product-specific regulations for vaping products that set out labelling requirements using authorities set out in the TVPA and the CCPSA make the regulatory framework easier to understand for industry as well as for Health Canada officials engaged in compliance and enforcement activities.
Regulatory analysis
Benefits and costs
A cost-benefit analysis (CBA) was commissioned by Health Canada to quantify the expected costs and benefits of the Regulations. The CBA was finalized in March 2018 and was based on market data collected in 2016. footnote 22 In response to a rapidly evolving vaping product market, and concerns that had been raised by industry, a supplemental cost analysis was completed in March 2019. The supplemental analysis focused solely on providing updated cost information related to the availability of child-resistant refillable vaping devices and included updated text for specific sections of the 2018 report. The CBA report and the supplementary cost-analysis report are available upon request to the departmental contact.
The supplementary report provided the accounting statement for 30 years, from 2016 to 2046, as shown in Table 1. The table reflects the central case for each industry cost category: labelling of vaping substances, labelling of vaping devices and their parts, child-resistant vaping substance containers and child-resistant refillable vaping devices and their parts for testing. Using information from the 2018 CBA report, an additional cost line for redesign was added for refillable vaping devices and their parts. Table 1 presents the estimated costs in 2016 Canadian dollars and uses a 7% discount rate for the 30-year projection. The statement shows the estimated annualized average cost to industry to comply with the requirements of the Regulations of $1,013,500, and the estimated total present value cost of $12,580,500.
The accounting statement also shows the estimated costs to Government for compliance promotion, monitoring and enforcement of the Regulations. There was greater certainty for the government costs, and these were reflected in the 2018 CBA report only as central values. The statement shows the estimated annualized average cost to Government to promote, monitor and enforce the Regulations of $223,000, and the estimated total present value cost of $2,965,000.
The statement shows the estimated annualized average total cost of the Regulations as $1,236,500, and the estimated total present value cost of $15,545,500.
Positive and negative impacts of the Regulations on consumers and industry, which were quantified but not monetized, are also listed in Table 1 as well as qualitative impacts.
Accounting Statement |
Year 1 |
Year 2 |
Year 3 |
Years 4 Through 30 |
Total PV |
Annualized Average |
|
|---|---|---|---|---|---|---|---|
A. Quantified impacts (2016 Canadian dollars, 7 % discount rate, 30-year project life) |
|||||||
Costs |
|||||||
Costs to industry |
Labelling of vaping substances |
3,159,500 |
0 |
0 |
0 |
3,159,500 |
254,500 |
Labelling of vaping devices and parts |
75,000 |
0 |
0 |
0 |
75,000 |
6,000 |
|
Child-resistance for vaping substances |
58,000 |
62,000 |
65,000 |
68,000 |
886,000 |
71,000 |
|
Child-resistance for refillable vaping devices, testing |
3,460,000 |
0 |
0 |
0 |
3,460,000 |
279,000 |
|
Child-resistance for refillable vaping devices, redesign |
5,000,000 |
0 |
0 |
0 |
5,000,000 |
403,000 |
|
Subtotal |
11,752,500 |
62,000 |
65,000 |
68,000 |
12,580,500 |
1,013,500 |
|
Costs to Government |
Labelling requirements: compliance promotion, monitoring and enforcement |
134,000 |
248,000 |
248,000 |
248,000 |
2,818,000 |
212,000 |
Child-resistance requirements: compliance promotion, monitoring and enforcement |
26,000 |
28,000 |
28,000 |
28,000 |
147,000 |
11,000 |
|
Subtotal |
160,000 |
276,000 |
276,000 |
276,000 |
2,965,000 |
223,000 |
|
Total costs |
11,912,500 |
338,000 |
341,000 |
344,000 |
15,545,500 |
1,236,500 |
|
B. Quantified impacts not monetized |
|||||||
Positive impacts |
Public health benefits are expected from reducing poisoning incidents and fatalities, especially to young children who may gain access to the vaping substances containing nicotine. A break-even analysis for the child-resistant container provisions suggests that avoiding one death every 24 to 92 years or one emergency room visit every 7 to 29 days may yield benefits commensurate to the costs estimated for the child-resistant container requirements. |
||||||
Negative impacts |
Consumers: Industry compliance costs may be passed through to consumers in the form of higher vaping substance and device prices, although the anticipated percentage increase in retail price is minor. |
||||||
Industry: Lost producer surplus for manufacturers and importers of vaping substances and devices, although these losses appear minor in comparison to industry revenue. Compliance costs for vaping substances are less than 0.5 % of industry revenue. Based on 2016 data, the CBA report stated that should there be significant costs associated with redesigning refillable vaping devices and parts and should these costs result in a lack of available compliant products upon coming into force, retail vape shops could experience a loss in profits that could be as high as 43 million dollars. However, there is a large degree of uncertainty in this estimate and the costs for redesign have more recently been assessed as negligible in the 2019 supplementary cost-analysis report based on the assumption that a wide range of devices will meet the child-resistant container requirements in the Regulations and are now globally available. Based on extensive research and consultation with testing laboratories, Health Canada believes there is indeed a wide range of devices that are now globally available and that have a high likelihood of meeting the child-resistant container requirements. Should retail vape shops begin sourcing such refillable child-resistant devices early, there should be minimal risk of profit loss. |
|||||||
C. Qualitative impacts |
|||||||
Positive impacts |
Consumers: The toxicity warning explains the toxicity exposure route in simple language and may improve consumers’ understanding that the acute toxicity risk posed by nicotine is related to ingesting it, not inhaling it. The nicotine concentration statement and the addictiveness warning are expected to increase consumers’ awareness of the presence of nicotine in certain vaping products and that nicotine is highly addictive. This information could assist consumers, in particular non-smokers and youth, in making an informed choice regarding the use of these products. Initiation of vaping by non-smokers and youth could potentially lead to nicotine addiction and a transition to tobacco product use. However, a slight decrease in the initiation rate of vaping products among non-smokers and youth could result in public health benefits. The bulk of these potential public health benefits would be derived from reducing the number of deaths attributable to cigarette use and exposure to second-hand smoke over a 30-year period. |
||||||
Industry: The Regulations streamline the regulatory framework by combining the labelling requirements from the TVPA and the CCPSA into a single, product-specific regulation that may make it easier for industry to understand how to comply. The Regulations will result in the new labelling requirements authorized by different Acts coming into force at the same time and this may enable industry to adjust their labels only once. |
|||||||
Negative impacts |
Consumers: Some consumers may experience a loss in economic welfare as a result of a reduction in the variety of vaping products available on the market. Aversion to health and safety messaging could potentially reduce consumer welfare. A loss of consumer surplus may occur if companies remove refillable vaping devices from the market or increase their prices in response to the requirements related to child resistance. |
||||||
Industry: Child-resistant container requirements for refillable vaping devices may hasten consumer migration to closed devices, imposing negative impacts on vape shops and small vaping substance manufacturers. Some closures among small vaping substance manufacturers are possible. |
|||||||
Costs
In Canada, vaping devices are almost exclusively imported, with China being the largest producer. This is not the case with vaping substances. Most vaping substances are manufactured in Canada, with the United States being the main source of imported substances. The estimation of costs to industry is challenging because the vaping industry is relatively new and continues to evolve. The CBA found that the information on industry characteristics was limited and that market conditions are rapidly evolving. Further changes in the vaping market are anticipated. These uncertainties have implications for the CBA, which are outlined below:
- Quantitative estimates of industry compliance costs were provided as lower- and upper-bound values, with the resulting broad ranges reflecting the considerable uncertainty in the underlying parameters.
- When reliable information was lacking, qualitative analysis was used to characterize the anticipated key outcomes. This was especially true for the assessment of the secondary impacts.
- Estimates were based on a snapshot of the industry as it existed in 2016. A variety of factors may influence the evolution of the vaping industry and the long-term outcome is difficult to forecast. To address recent changes in the availability of child-resistant devices and update relevant cost estimates, a supplemental CBA was completed in March 2019.
- The analysis does not differentiate between costs that would be carried by foreign suppliers of vaping products and costs that would be carried by domestic producers.
Several assumptions were made in the CBA, including
- an analytical period of 30 years spanning from 2016 to 2046 was adopted;
- an annual discount rate of 7%;
- a base year of 2016 to which costs are discounted;
- monetized values are expressed in 2016 Canadian dollars; and
- the costs carried by foreign suppliers would be passed on to Canadian importers.
The CBA estimated the costs to industry across four elements of the Regulations: labelling of vaping substances, labelling of vaping devices and their parts, child-resistant vaping substance containers and child-resistant vaping devices and their parts.
The 2018 and 2019 CBA reports provided lower- and upper-bound values for costs to industry across the labelling and the child-resistance requirements of the Regulations. Health Canada has selected a simple average, lower- or upper-bound value as the central value for each industry cost category based on the Department’s understanding of the most likely outcomes for industry. An explanation of the central value applied to each cost category is provided in the following sections.
Compliance for labelling of vaping substances
The central value for the annualized average cost to industry to implement the labelling requirements for stand-alone containers of vaping substances was estimated to be $254,500, and the total present value cost was estimated to be $3,159,500. A mid-range cost estimate was generated by calculating a simple average from the lower- and upper-cost estimates, which were $55,000 and $454,000 for the annualized average, and $689,000 and $5,630,000 for the present value. A mid-range cost estimate was applied, given that industry has already adjusted to the labelling requirements for the toxicity warning and nicotine content, as applicable, since these requirements have been in place, under the CCCR, 2001 provisions, as of May 23, 2018. The mid-range estimate reflects the label redesign and printing that may be required to comply with labelling elements that are new. A new element for all products is the ingredient list. For products that contain nicotine, the new elements are the nicotine concentration statement, the nicotine addictiveness warning, and the modified toxicity warning. Therefore, industry would bear some costs. It is estimated that the costs would be centralized between the low and high estimates provided in the CBA.
Compliance for labelling of vaping devices and their parts
The central value for the annualized average cost to industry to implement the labelling requirements for vaping devices and their parts was estimated at $6,000, and the total present value cost was estimated at $75,000. These central values were identified using the upper estimates from the ranges for this cost category: $1,000 and $6,000 for the annualized average, and $17,000 and $75,000 for the present value. While some prefilled vaping devices and their parts may already have labels that present an ingredient list, the nicotine concentration and warnings about the addictiveness of nicotine, as applicable, some label or package redesign may be required. It is appropriate to use the upper-cost estimates for these labelling requirements since they are new for industry.
Compliance for child-resistant stand-alone containers of vaping substances
The central value for the annualized average cost to industry to implement the child-resistant container requirements for stand-alone containers of vaping substances containing nicotine was estimated at $71,000, and the total present value cost was estimated at $886,000. These central values were identified using the lower estimates from the ranges for this cost category: $71,000 and $301,000 for the annualized average, and $886,000 and $3,739,000 for the present value. It is appropriate to use the low-cost estimates for the child-resistant containers of vaping substances containing nicotine, given that the CBA was completed prior to royal assent of the Act, when there was no Canadian requirement for child-resistant containers. The child-resistant container requirements for stand-alone containers of vaping substances containing nicotine as described in the Regulations are currently in effect and have been enforced. For example, after May 23, 2018, Health Canada contacted 269 establishments across Canada and inspected 497 stand-alone containers of vaping substances containing nicotine as part of an initial compliance and enforcement project related to the child-resistant container requirements of the CCCR, 2001. The results of the inspections showed 95.4% compliance with the child-resistant container requirements. Therefore, the lower-cost estimate was applied for the central value in this cost category, since the majority of products on the market are already in compliance.
Compliance for child-resistant refillable vaping devices and their parts — Testing
The central value for the annualized average cost to industry to implement the requirements for refillable tanks of vaping devices and their parts that may hold a vaping substance to be child resistant was estimated at $279,000, and the total present value cost was estimated at $3,460,000. These central values were identified using the upper estimates from the ranges for this cost category: $28,000 and $279,000 for the annualized average, and $346,000 and $3,460,000 for the present value. It is appropriate to use the upper cost estimates for the child-resistant container requirements for refillable tanks of vaping devices and their parts, since these requirements are new for industry and they will be required to test the devices or parts to one of the child test protocols identified in the Regulations.
Compliance for child-resistant refillable vaping devices and their parts — Redesign
The present value of the cost to industry to redesign refillable child-resistant vaping devices and their parts was estimated to be about $5,000,000, based on an estimate of the number of models to be redesigned (10) and the estimated redesign cost of $500,000 per model. The annualized average cost to industry related to redesign was $403,000. The total present value cost was estimated to be $5,000,000. These values were derived by applying the lower estimates from the ranges for this cost category ($500,000 to $1,000,000 per model) to an illustrative estimate of non-compliant models of 10.
The CBA report estimated in 2016 that the number of refillable vaping devices on the Canadian market that would require redesign to meet the child-resistant container requirements was between 67 and 168, and the speculated redesign cost was between $500,000 and $1,000,000 per model. Recognizing that vaping devices are almost exclusively imported in Canada, a supplemental cost analysis, completed in March 2019, was undertaken to provide updated cost information related to the global availability of child-resistant refillable vaping devices. The analysis focused on vaping device brands popular on the Canadian market. Relevant devices on retail websites were identified to draw a comparison between similarly designed products that were child resistant compared to products that were not child resistant. Similarity was based on the product’s category, style, tank capacity, coil-resistance and battery wattage. The supplemental analysis indicated that child-resistant devices were not more or less expensive than comparable devices that were not child resistant. They were relatively cost comparable. The supplemental report concludes that it is no longer the case that device makers may need to invest in complex redesigns and coordinate the implementation of these new designs with overseas manufacturers. Several suppliers have different refillable models that are indicated as being child resistant.
Health Canada has applied an estimate of 10 models that would require redesign. This low estimate is supported by the results of the 2019 supplemental cost analysis described above. Further, it is supported by the results of an online market search that was completed in October 2019. The search found over 150 models of vaping devices or their parts with child-resistant designs. These were available in a range of tank volumes and popular brands that are already carried by Canadian importers, distributors and retailers. These designs are likely to meet the child-resistant container requirements set out in the Regulations; however, importers would need to verify this by either obtaining a test report from the supplier or arranging for testing to verify compliance. These estimated testing costs were already discussed in the previous section. Given that child-resistant devices and their parts are available on the global marketplace and the costs for developing these designs have already been incurred, a small number of models was used in the cost estimate to reflect that it is possible that some industry members may still choose to redesign products to enhance consumer choice for the Canadian market.
It is appropriate to use the lower cost estimates for the child-resistant container requirements for refillable vaping devices and their parts for the following reasons. The estimated $750,000 redesign cost per model noted in the CBA report was based on one industry member’s speculation in 2016. This estimate was effectively deleted from the CBA report through the 2019 supplemental cost analysis, which provided annotations for several parts of the 2018 report related to costs for child-resistant devices. Even though these requirements are new for Canada, they were first published in European Union legislation in 2014 and implemented in European Union Member States in 2016. The speculated $750,000 cost reflected a need to invest in complex redesigns and coordinate implementation of these new designs with overseas manufacturing operations. Since child-resistant devices and their parts already exist, complex redesigns are not necessary and the lower cost estimate of $500,000 is appropriate.
Costs to Government
Costs to the Government include the costs of compliance promotion, compliance monitoring and enforcement actions. Initial implementation costs for child-resistant containers and labelling compliance activities are estimated to be $160,000 and annual costs at $276,000 in the first five years and $180,000 per year afterwards. The annualized average cost for the administration of the child-resistant container and labelling requirements is estimated at $223,000, and the present value cost is estimated at $2,965,000. It is noted that Health Canada has been actively promoting, monitoring and enforcing several elements of the Regulations already in effect under the CCPSA since royal assent of the Act. As a result, the Government may have already assumed many of the initial costs.
Costs to consumers
No quantified direct costs to consumers were indicated by the cost-benefit analysis. Major identified impacts affecting consumers included a possible lack of product diversity, both in terms of vaping substances and vaping devices. This was related to the assumption that increasing costs of compliance could see a decrease in product diversity both directly, from higher costs of compliance, and indirectly, due to a possible decrease in the size of industry. Another major impact identified was the possible higher cost of vaping products due to compliance costs being passed on to the consumer.
Benefits
Many of the same challenges of analyzing costs were present when attempting to calculate potential benefits of the Regulations. Therefore, a quantitative estimate of the direct impact to the Regulations was not possible. The CBA presents several illustrative benefits analyses.
The requirements under the authority of the TVPA to display information on vaping products and their packaging enhance awareness of the health hazards posed by using vaping products and can prevent the public from being deceived or misled with respect to these health hazards. By informing the people of Canada about nicotine concentrations in a consistent manner, and by making them aware that nicotine is highly addictive, it is expected that the requirements will contribute to increasing awareness about the addictiveness of vaping products and enable Canadians to make an informed choice regarding the use of these products, including avoiding exposure to nicotine. The labelling requirements will result in public health benefits when they assist youth and non-users of tobacco products to avoid vaping product use and when they contribute to an adult smokers’ decision to completely switch to vaping.
A relatively small effect on the initiation of vaping product use would be sufficient to produce public health benefits equivalent to or greater than the estimated costs. The bulk of the public health benefits stem from a reduction of deaths attributable to cigarette use and exposure to second-hand smoke over a 30-year period.
The requirements under the authority of the CCPSA to prohibit vaping products containing very toxic concentrations of nicotine, and to require child-resistant containers and labelling on products to warn that nicotine is toxic when ingested, will provide benefits by helping to protect against poisoning incidents and fatalities, especially among young children who may gain access to the products and their contents in a household. Given that child-resistant containers are not childproof, the labelling, which includes the toxic hazard symbol, is important as a consistent reminder for parents and caregivers to securely close the container, and to keep it stored out of sight and reach of children. Health Canada encourages parents, caregivers and educators to teach children at an early age that the hazard symbol means “Danger. Don’t touch it.” footnote 23
The general success of child-resistant container requirements in decreasing childhood poisoning is discussed in the Pediatric Poisoning Fatalities from 1972 through 2014 report prepared by the Consumer Product Safety Commission of the United States. footnote 24 The report states that the year after the Poison Prevention Packaging Act came into force, there was a marked decrease in pediatric poisoning incidents. Noting that the report only considers fatalities, it shows that five years immediately after the Act came into force, pediatric fatalities had dropped by 56%. Compared to 216 pediatric poisoning fatalities in 1972, the rates continued to drop each year to the long-term average of approximately 27 pediatric fatalities a year in 2014. Fatalities are the most extreme consequence of poisoning incidents; the number of poisoning incidents themselves would have been substantially higher.
The model used in the CBA to work out a breakeven analysis for the child-resistant container provisions applied the value of a statistical life footnote 25 and estimated an emergency room visit to cost $6,560 per incident. The CBA concluded that avoiding one death every 24 to 92 years or one emergency room visit every 7 to 29 days would be equivalent to the annual costs associated with implementing the child-resistant container provisions in the Regulations.
Small business lens
The small business lens applies, as there are impacts on small businesses associated with the Regulations.
Table 2 presents a summary of costs estimated for small businesses in Canada as a result of the Regulations. It is assumed that 99% of the 2 250 vaping businesses, or approximately 2 228, are small businesses that are impacted by the Regulations. The table presents the estimated costs in 2016 Canadian dollars and uses a 7% discount rate for the 30-year projection. The administrative costs were estimated using 2012 dollars and converted to 2016 dollars using the Bank of Canada inflation calculator. footnote 26
Number of small businesses impacted |
2 228 |
|---|---|
Number of years |
30 years |
Base year for costing |
2016 |
Compliance Costs |
Annualized Value ($) |
Present Value ($) |
|---|---|---|
Labelling of vaping substances |
251,955 |
3,127,905 |
Labelling of vaping devices and parts |
5,940 |
74,250 |
Child resistance for vaping substances |
70,290 |
877,140 |
Child resistance for refillable vaping devices |
675,180 |
8,375,400 |
Total |
1,003,365 |
12,454,695 |
Administrative Costs |
Annualized Value ($) |
Present Value ($) |
Child-resistance documentation for vaping devices (only applicable to device manufacturers and importers). Assume 50 businesses, and 45 small businesses. |
3,151 |
56,499 |
Total |
3,151 |
56,499 |
Total cost (all impacted small businesses) |
607,546 |
7,561,194 |
Cost per impacted small business |
273 |
3,394 |
At the time Health Canada conducted consultations on the Regulations and at the time the CBA was developed, the vaping industry was dominated by small businesses. Health Canada has modified the Regulations in response to the comments received during the consultations, POR and CBA processes. The modifications were intended to reduce the compliance burden on small businesses associated with the cost of changing and implementing the new product labels, as well as the costs associated with the child-resistant requirements for refillable vaping devices and their parts.
The coming into force of the exemption set out in subsection 4(4) of the CCPSA delayed the implementation of the child-resistant requirement for refillable vaping devices and their parts, and has allowed time for industry to source products to meet this requirement.
Health Canada’s plan for a joint regulation that sets out labelling requirements using authorities under the CCPSA and the TVPA is another modification to benefit small businesses. This approach sets out all requirements in a single, product-specific regulation, therefore making the regulatory framework easier to understand. Health Canada has assessed the administrative burden that the Regulations will place on small businesses. This assessment was based on the compliance and enforcement program undertaken in 2018, and limited Canadian market intelligence. Health Canada is assuming that a large majority of vaping device manufacturers and importers are small businesses as defined by Statistics Canada. The CBA that was completed in March 2018 identified that there were an estimated 30 to 50 device manufacturers or importers active in the Canadian market. Assuming the upper range for the number of manufacturers and importers, Health Canada estimates that 90%, or 45 small businesses, will be affected. The supplemental CBA report completed in March 2019 indicated that there is a range of between 67 and 168 models of open vaping devices on the Canadian market. This can be averaged to a midrange estimate of three vaping devices per manufacturer or importer.
One-for-one rule
The one-for-one rule applies since there is an incremental increase in the administrative burden on business, and a new regulatory title (title in) is being introduced. The Regulations include a requirement for importers and manufacturers to obtain and maintain records to demonstrate that containers of vaping substances containing nicotine meet the child test protocol in a prescribed standard. This requirement is currently in force for stand-alone containers of vaping substances that contain nicotine. The Regulations set out these requirements for refillable vaping devices and refillable vaping device parts that may contain vaping substances containing nicotine. As a result, the Regulations impose a new administrative burden on manufacturers and importers of vaping devices or their parts.
For the purposes of this section, it has been assumed each manufacturer or importer of vaping devices has three vaping devices that require testing, and that testing is completed every three years. In this case, the activities related to record keeping are contracting a testing facility (approximately 2 hours/year), maintaining a test report (approximately 30 minutes/year) and producing a test report upon the request of an inspector (approximately 30 minutes/year). It is assumed that the staff level involved with contracting activities and producing a test report for inspection and enforcement purposes would be management, at a labour rate of $46.26 per hour (Statistics Canada, Labour Force Survey). Clerical duties involved with maintaining a test report, such as storing, copying and distributing are calculated at a labour rate of $25.30 per hour (Statistics Canada, Labour Force Survey). The annualized administrative cost (discounted to 2012 and expressed in 2012 Canadian dollars) is estimated to be $2,998 for industry as a whole, or $60 per business.
The labelling portion of the Regulations will not increase the administrative burden on businesses as there are no associated reporting or record-keeping requirements.
Regulatory cooperation and alignment
Both the European Union and the United States require vaping products containing nicotine to have specific information on the product label. In addition to other provisions, the European Union requires a warning about the addictiveness of nicotine, a list of ingredients and information on the toxicity of nicotine. The United States Food and Drug Administration requires a nicotine addictiveness statement to be displayed on products containing nicotine and recommends that a nicotine exposure warning be displayed on products to help prevent poisoning.
With regard to the nicotine addictiveness statement, the exact wording required by the European Union and the United States is different. Both their statements were tested by Health Canada during the POR. The longer warning used in the United States was preferred in the POR testing since it presents a clear link between the presence of nicotine and the addictiveness statement. However, in consideration of the limited space for labelling on vaping products and in order to respect Canada’s official language requirements, the shorter nicotine addictiveness statement (i.e. “WARNING: Nicotine is highly addictive” and “AVERTISSEMENT : La nicotine crée une forte dépendance” in French) co-located on the main display panel with the nicotine concentration statement is required.
The Regulations require vaping devices and their parts to be child-resistant, as well as stand-alone containers of vaping substances containing nicotine in a concentration of 0.1 mg/mL or more to be child-resistant. This is similar to requirements in the European Union, whereas the United States only requires stand-alone containers of vaping substances containing nicotine to be child-resistant.
Strategic environmental assessment
In accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals, a preliminary scan concluded that a strategic environmental assessment is not required.
Gender-based analysis plus
At this time, given that vaping products were introduced to the global marketplace just over a decade ago, the potential short and long-term effects of vaping remain unknown. The labelling requirements provide information about the known health hazards linked to nicotine, namely its addictiveness and its toxicity when ingested. This information is relevant to all people of Canada, irrespective of gender-based analysis plus (GBA+) considerations.
The child-resistant container requirements help to protect young children from ingesting a vaping substance containing toxic concentrations of nicotine. They consider the impact of age. Young children, due to their curiosity, oral exploration and their low body weights, are particularly vulnerable to nicotine poisoning through ingestion. The nicotine concentration limit of 0.1 mg/mL, which triggers the child-resistant container and toxicity warning requirements, was identified considering the body weight of young children. The requirements help to protect young children from gaining access to vaping substances containing toxic concentrations of nicotine irrespective of their height, weight, age or sex. The child-resistant container test protocol assesses the abilities of young children of an even distribution of sex and age in two-month intervals for children between the ages of 42 and 52 months. While the child test protocol requires test subjects to be healthy with no evident disability that may affect their manual dexterity, the effect of this qualification is to provide protection for children in the full range of manual dexterity abilities.
Rationale
Vaping products present both a challenge and an opportunity for public health in Canada. Vaping products are harmful, particularly to the health of youth and non-users of tobacco products. For adult tobacco users (e.g. smokers) who completely switch to vaping, these products offer a less harmful alternative to tobacco use.
These Regulations require vaping products, which contain nicotine, to display information about their nicotine concentration and about the addictiveness of nicotine. Health warnings have long been used as a means to educate consumers about the risks associated with harmful products. The nicotine concentration statement and the health warnings are intended to enhance awareness of the health hazards posed by using vaping products and to prevent the public from being deceived or misled with respect to these health hazards. In addition, the Regulations set out permitted expressions that may be used on the product or package to indicate when a vaping product is without nicotine. This information will allow the consumer to make an informed choice about the products that they choose to use.
In 2017, Health Canada consulted the public on how to regulate vaping products in Canada. Health Canada provided 10 measures for consideration, 4 of which pertain to the labelling elements of the Regulations. The Regulations require that vaping products containing nicotine must have a health warning that informs about the addictiveness of nicotine and have a standardized nicotine concentration statement. All vaping substances must display a list of ingredients. The Regulations contain measures that align Canadian policy for required labelling on vaping substances containing nicotine with policy objectives in the United States and the European Union.
In 2017, Health Canada also stated that, in application of the Act, the CCPSA would pertain to vaping products that are not marketed for a therapeutic use. Vaping substances containing nicotine would be specifically regulated by the CCCR, 2001 to help protect young children against the acute poisoning risk. These Regulations would supplement the obligations placed on regulated parties by sections 7 and 8 under the CCPSA concerning the prohibitions of the manufacture, importation, advertisement or sale of a consumer product that is a danger to human health or safety. However, these were accepted as interim measures that would be applied until product-specific regulations could be developed. The Regulations implement these health and safety measures in an accessible and transparent manner.
A CBA determined that the costs to industry associated with compliance with the child-resistant container and labelling provisions contained within the Regulations are estimated at $610,500 on an annualized average basis.
The impact on industry is expected to be minor. Industry has been aware, since October 2017, of Health Canada’s potential options for vaping product regulation. The measures under consideration at the time and presented in the consultation were generally well received by all stakeholders. International trading partners already require similar provisions be met for nicotine concentration and addictiveness warnings as well as for an ingredient list. A preliminary Health Canada compliance and enforcement program demonstrated a high degree of compliance with the child-resistant requirements on stand-alone containers of vaping substances containing nicotine. Prior to 2017, industry members indicated that requiring devices to be child-resistant would have a significant impact on their costs. However, limited market intelligence collected in 2019 has shown that vaping devices that are child-resistant are available at comparable costs.
Implementation, compliance and enforcement, and service standards
The Regulations are made under the authority of both the TVPA and the CCPSA, and clearly set out which prohibitions or requirements exist under which act’s authority. The requirements of the Regulations come into force on July 1, 2020, including the requirements for stand-alone containers of vaping substances containing nicotine to be child-resistant. There is one exception. The cominginto-force date related exclusively to the child-resistant container requirements for refillable vaping devices and their parts is January 1, 2021. The interim application of the CCCR, 2001 continues for vaping substances containing nicotine that are subject to the CCPSA until July 1, 2020.
Until July 1, 2020, a stand-alone container of vaping substance containing nicotine may meet the toxicity warning requirements in the Regulations or those in the CCCR, 2001.
As a complementary instrument to the Regulations, Health Canada will continue to engage in proactive outreach to industry members including manufacturers, importers, distributors and retailers to assist them in understanding and complying with the new requirements. For example, to address concerns raised by industry in their ability to comply with child-resistant container requirements for refillable devices and their parts, information and educational materials will be developed to reinforce their responsibilities. These materials will be distributed to industry to inform them of the changes, and opportunities to seek clarification on the requirements will be provided. The testing methods used by Health Canada to determine nicotine concentration in vaping substances are available to facilitate compliance as well as verification.
The Regulations will not result in any major changes to Health Canada’s compliance and enforcement activities. Compliance and enforcement activities related to the Regulations will follow established Health Canada approaches and procedures, including sampling and testing of products, inspections at retail locations, followup on incidents reported by the Canadian public and public health organizations, as well as followup on mandatory incident reports by industry. Non-compliant products will be subject to the actions available to Health Canada inspectors under the appropriate powers of either the TVPA or CCPSA. For the requirements under the authority of the TVPA, appropriate measures would be taken, which could include warning letters, negotiated compliance, seizures and possible prosecution. Actions under the CCPSA may include a voluntary commitment to product correction by industry, negotiation with industry for the voluntary removal of non-compliant products from the market, seizure, orders for recall or other measures, administrative monetary penalties, and possible prosecution.
A compliance monitoring and enforcement program will likely be initiated within six months to one year after the requirements come into force.
Contact
Rob Graham
Consumer and Hazardous Products Safety Directorate
Healthy Environments and Consumer Safety Branch
Health Canada
Address Locator: 4908B
269 Laurier Avenue West
Ottawa, Ontario
K1A 0K9
Fax: 613‑952‑2551
Email: rob.graham@canada.ca