Canada Gazette, Part I, Volume 151, Number 52: GOVERNMENT NOTICES
December 30, 2017
DEPARTMENT OF CITIZENSHIP AND IMMIGRATION
IMMIGRATION AND REFUGEE PROTECTION ACT
Ministerial Instructions with respect to the processing of applications for a permanent resident visa made by parents or grandparents of a sponsor as members of the family class and the processing of sponsorship applications made in relation to those applications
These Instructions are published in the Canada Gazette in accordance with subsection 87.3(6) of the Immigration and Refugee Protection Act (Act).
These Instructions are given, pursuant to section 87.3 and subsections 92(1.1) and (2) of the Act, by the Minister of Citizenship and Immigration as, in the opinion of the Minister, these Instructions will best support the attainment of the immigration goals established by the Government of Canada by seeing families reunited in Canada. By using a randomized selection process, sponsors will have the same opportunity of having their application accepted for processing within the 10 000 sponsorship applications accepted for processing in any year. As part of the randomized selection process, a period of time will be provided to persons to indicate their interest in making a sponsorship application to sponsor their parents or grandparents. Before 10 000 persons are randomly selected, duplicate entries will be removed, keeping only the most recent entry of a person. Finally, invitations to make a sponsorship application sent by the Department of Citizenship and Immigration (Department) are not transferable, further ensuring fairness in the management of intake into the parent and grandparent program.
Scope
These Instructions apply to applications for a permanent resident visa of sponsors' parents or grandparents made under the family class, referred to in paragraphs 117(1)(c) and (d) of the Immigration and Refugee Protection Regulations (Regulations), respectively, as well as to sponsorship applications made in relation to those applications.
Interpretation
For the purposes of these Instructions,
- (a) a working day does not include Saturdays or holidays within the meaning of subsection 35(1) of the Interpretation Act, and if New Year's Day falls on a Saturday or a Sunday, a working day also does not include the following Monday; and
- (b) the period during which a person can indicate their interest in making a sponsorship application begins at noon Eastern standard time on the first working day of a calendar year and ends at noon Eastern standard time on the 30th day following the first working day.
Number of applications to be accepted for processing in a year
A maximum of 10 000 sponsorship applications made in relation to applications for a permanent resident visa, which are made by sponsors' parents or grandparents under the family class, are accepted for processing each year. The year begins on January 1 and ends on December 31 of the same calendar year.
Conditions — sponsorship applications
With respect to a year, in order to be processed, any sponsorship application referred to in these Instructions that has not been returned under section 12 of the Regulations for not meeting the requirements of sections 10 and 11 of the Regulations — for example by not using all the applicable forms provided by the Department in the application package published on the website of the Department or by not including all information, documents and evidence referred to in paragraph 10(1)(c) of the Regulations — must meet the following conditions:
- (a) the sponsorship application is made by a person who, having indicated — during the period during which they could do so — their interest in making a sponsorship application by means that have been made available by the Department for that purpose, has been invited to make the application after they were randomly selected by the Department among the other interested persons;
- (b) the sponsorship application has been received by the Department within the period of 60 days after the day on which the Department sent the sponsor an invitation to make a sponsorship application;
- (c) the sponsorship application indicates the same information, such as name, date of birth, address, country of birth, as that of the person who has been invited to make such an application; and
- (d) the sponsorship application is accompanied by the documents required by the application package published on the website of the Department, as amended from time to time.
Conditions — permanent resident visa applications
With respect to a year, in order to be processed, any permanent resident visa application referred to in these Instructions that has not been returned under section 12 of the Regulations for not meeting the requirements of sections 10 and 11 of the Regulations — for example by not using all the applicable forms provided by the Department in the application package published on the website of the Department or by not including all information, documents and evidence referred to in paragraph 10(1)(c) of the Regulations — must meet the following conditions:
- (a) the permanent resident visa application is made by an applicant sponsored by a person who, having indicated — during the period during which they could do so — their interest in making a sponsorship application by means that had been made available by the Department for that purpose, has been invited to make a sponsorship application after they were randomly selected by the Department among the other interested persons;
- (b) the permanent resident visa application is made by an applicant being sponsored by a person whose sponsorship application has been received by the Department within the period of 60 days after the Department sent them an invitation to make a sponsorship application;
- (c) the permanent resident visa application has been received by the Department within the period of 60 days referred to in paragraph (b); and
- (d) the permanent resident visa application is accompanied by the documents required by the application package published on the website of the Department, as amended from time to time.
Order for processing
Applications meeting the applicable conditions established by these Instructions are processed in the order in which they are received by the Department.
Humanitarian and compassionate requests
A request made under subsection 25(1) of the Act from outside Canada and that accompanies an application that was not accepted for processing under these Instructions will not be processed.
Disposition of applications
Any application that does not meet the applicable conditions established by these Instructions will be returned.
Repeal
The following Instructions are repealed, effective January 1, 2018:
- Ministerial Instructions with respect to the processing of applications for a permanent resident visa made by parents or grandparents of a sponsor as members of the family class and the processing of sponsorship applications made in relation to those applications, published in the Canada Gazette, Part I, on January 7, 2017.
Coming into effect
These Instructions take effect on January 1, 2018.
Ottawa, December 20, 2017
Ahmed Hussen, P.C., M.P.
Minister of Citizenship and Immigration
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Notice with respect to reporting of greenhouse gases (GHGs) for 2017
Notice is hereby given, pursuant to subsection 46(1) of the Canadian Environmental Protection Act, 1999 (the Act), that, with respect to emissions of GHGs identified in Schedule 1 to this notice and for the purpose of conducting research, creating an inventory of data, formulating objectives and codes of practice, issuing guidelines or assessing or reporting on the state of the environment, any person who operates a facility described in Schedule 3 to this notice during the 2017 calendar year, and who possesses or who may reasonably be expected to have access to information described in Schedules 4 through 11 to this notice, shall provide the Minister of Environment with this information no later than June 1, 2018.
Persons subject to this notice shall address responses or enquiries to the following address:
Greenhouse Gas Reporting Program
Pollutant Inventories and Reporting Division
Environment and Climate Change Canada
Place Vincent Massey, 7th Floor
351 Saint-Joseph Boulevard
Gatineau, Quebec
K1A 0H3
Telephone: 819-938-3258 or 1-877-877-8375
Email: ec.ges-ghg.ec@canada.ca
This notice applies to the calendar year 2017. Pursuant to subsection 46(8) of the Act, persons subject to this notice shall keep copies of the information required under this notice, together with any calculations, measurements and other data on which the information is based, at the facility to which the calculations, measurements and other data relate, or at the facility's parent company, located in Canada, for a period of three years from the date the information is required to be submitted. Where the person chooses to keep the information required under the notice, together with any calculations, measurements and other data, at the facility's parent company in Canada, that person shall inform the Minister of the civic address of that parent company.
If a person who operates a facility with respect to which information was submitted in response to the Notice with respect to reporting of greenhouse gases (GHGs) for 2016 determines that the facility is not required to provide the information set out in Schedules 4 through 11 of this notice, the person shall notify the Minister of the Environment that the facility does not meet the criteria set out in Schedule 3 of this notice, no later than June 1, 2018.
The Minister of the Environment intends to publish information on greenhouse gas emission totals by gas by facility submitted in response to this notice. Pursuant to section 51 of the Act, any person who provides information in response to this notice may submit, with their information and no later than their deadline for submission, a written request that it be treated as confidential based on the reasons set out in section 52 of the Act. The person requesting confidential treatment of the information shall indicate which of the reasons in section 52 of the Act applies to their request. Nevertheless, the Minister may disclose, in accordance with section 53 of the Act, information submitted in response to this notice. Every person to whom a notice is directed shall comply with the notice. A person who fails to comply with the Act is subject to the offence provision.
Jacqueline Gonçalves
Director General
Science and Risk Assessment Directorate
On behalf of the Minister of the Environment
SCHEDULE 1
Greenhouse Gases
| Greenhouse Gas | Formula | CAS Registry Number (see note 1) | 100-year Global Warming Potential (GWP) (see note 2) | |
|---|---|---|---|---|
| 1. | Carbon dioxide | CO2 | 124-38-9 | 1 |
| 2. | Methane | CH4 | 74-82-8 | 25 |
| 3. | Nitrous oxide | N2O | 10024-97-2 | 298 |
| 4. | Sulphur hexafluoride | SF6 | 2551-62-4 | 22 800 |
| Hydrofluorocarbons (HFCs) | ||||
| 5. | HFC-23 | CHF3 | 75-46-7 | 14 800 |
| 6. | HFC-32 | CH2F2 | 75-10-5 | 675 |
| 7. | HFC-41 | CH3F | 593-53-3 | 92 |
| 8. | HFC-43-10mee | C5H2F10 | 138495-42-8 | 1 640 |
| 9. | HFC-125 | C2HF5 | 354-33-6 | 3 500 |
| 10. | HFC-134 | C2H2F4 (Structure: CHF2CHF2) | 359-35-3 | 1 100 |
| 11. | HFC-134a | C2H2F4 (Structure: CH2FCF3) | 811-97-2 | 1 430 |
| 12. | HFC-143 | C2H3F3 (Structure: CHF2CH2F) | 430-66-0 | 353 |
| 13. | HFC-143a | C2H3F3 (Structure: CF3CH3) | 420-46-2 | 4 470 |
| 14. | HFC-152a | C2H4F2 (Structure: CH3CHF2) | 75-37-6 | 124 |
| 15. | HFC-227ea | C3HF7 | 431-89-0 | 3 220 |
| 16. | HFC-236fa | C3H2F6 | 690-39-1 | 9 810 |
| 17. | HFC-245ca | C3H3F5 | 679-86-7 | 693 |
| Perfluorocarbons (PFCs) | ||||
| 18. | Perfluoromethane | CF4 | 75-73-0 | 7 390 |
| 19. | Perfluoroethane | C2F6 | 76-16-4 | 12 200 |
| 20. | Perfluoropropane | C3F8 | 76-19-7 | 8 830 |
| 21. | Perfluorobutane | C4F10 | 355-25-9 | 8 860 |
| 22. | Perfluorocyclobutane | c-C4F8 | 115-25-3 | 10 300 |
| 23. | Perfluoropentane | C5F12 | 678-26-2 | 9 160 |
| 24. | Perfluorohexane | C6F14 | 355-42-0 | 9 300 |
- Note 1: The Chemical Abstracts Service (CAS) Registry Number is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
- Note 2: United Nations Framework Convention on Climate Change (UNFCCC), 2014. FCCC/CP/2013/10/Add.3. Decision 24/CP.19. Revision of the UNFCCC Reporting on annual inventories for Parties included in Annex I to the Convention, November 2013.
SCHEDULE 2
Definitions
The following definitions apply to this notice and its schedules:
- “2006 Intergovernmental Panel on Climate Change (IPCC) Guidelines” means the 2006 IPCC Guidelines for National Greenhouse Gas Inventories, prepared by the Intergovernmental Panel on Climate Change National Greenhouse Gas Inventories Program. (Lignes directrices 2006 du Groupe d'experts intergouvernemental sur l'évolution du climat (GIEC) pour les inventaires nationaux de gaz à effet de serre)
- “aluminium manufacturing” means primary processes that are used to manufacture aluminium from alumina, including electrolysis in prebake and Søderberg cells, anode and cathode baking for prebake cells, and green coke calcination. (fabrication d'aluminium)
- “biomass” means plants or plant materials, animal waste or any product made of either of these, including wood and wood products, charcoal, and agricultural residues; biologically derived organic matter in municipal and industrial wastes, landfill gas, bio-alcohols, black liquor, sludge digestion gas and animal- or plant-derived oils. (biomasse)
- “Canada's Greenhouse Gas Quantification Requirements” means Canada's Greenhouse Gas Quantification Requirements, Greenhouse Gas Reporting Program, Environment and Climate Change Canada, 2017. (Exigences relatives à la quantification des gaz à effet de serre du Canada)
- “carbon dioxide equivalent (CO2 eq.)” means a unit of measure for comparison between greenhouse gases that have different GWPs. (see footnote 1) [équivalent en dioxyde de carbone (éq. CO2)]
- “CAS Registry Number” means the Chemical Abstracts Service Registry Number. (numéro d'enregistrement CAS)
- “cement manufacturing” means all processes used to manufacture portland, ordinary portland, masonry, pozzolanic or other hydraulic cements. (fabrication de ciment)
- “CEMS” means Continuous Emission Monitoring Systems. (SMECE)
- “CKD” means cement kiln dust. (PFC)
- “CO2 capture” means the capture of CO2 at an integrated facility that would otherwise be released to atmosphere. (capture de CO2)
- “CO2 emissions from biomass decomposition” means releases of CO2 resulting from aerobic decomposition of biomass and from the fermentation of biomass. (émissions de CO2 provenant de la décomposition de la biomasse)
- “CO2 injection” means an activity that places captured CO2 into a long-term geological storage site or an enhanced fossil fuel recovery operation. (injection de CO2)
- “CO2 storage” means a long-term geological formation where CO2 is stored. (stockage de CO2)
- “CO2 transport system” means transport of captured CO2 by any mode. (installation de transport de CO2)
- “cogeneration unit” means a fuel combustion device which simultaneously generates electricity and either heat or steam. (unité de cogénération)
- “Continuous Emission Monitoring Systems” means the complete equipment for sampling, conditioning, and analyzing emissions or process parameters and for recording data. (systèmes de mesure et d'enregistrement en continu des émissions)
- “CSM” means cyclohexane-soluble matter. (MSC)
- “electricity generating unit” means any device that combusts solid, liquid, or gaseous fuel for the purpose of producing electricity either for sale or for use on-site. This includes cogeneration unit(s), but excludes portable or emergency generators that have less than 50 kW in nameplate generating capacity or that generate less than 2 MWh during the reporting year. (unité de production d'électricité)
- “emissions” means direct releases from sources that are located at the facility. (émissions)
- “enhanced fossil fuel recovery operation” means enhanced oil recovery, enhanced natural gas recovery and enhanced coal bed methane recovery. (opération améliorée de récupération des combustibles fossiles)
- “facility” means an integrated facility, a pipeline transportation system, or an offshore installation. (installation)
- “flaring emissions” means controlled releases of gases from industrial activities, from the combustion of a gas or liquid stream produced at the facility, the purpose of which is not to produce useful heat or work. This includes releases from waste petroleum incineration; hazardous emission prevention systems (in pilot or active mode); well testing; natural gas gathering systems; natural gas processing plant operations; crude oil production; pipeline operations; petroleum refining; chemical fertilizer production; steel production. (émissions de torchage)
- “fossil fuel production and processing” means the exploration, extraction, processing including refining and upgrading, transmission, storage and use of solid, liquid or gaseous petroleum, coal or natural gas fuels, or any other fuels derived from these sources. (production et transformation de combustibles fossiles)
- “fugitive emissions” means releases from venting, flaring or leakage of gases from fossil fuel production and processing; iron and steel coke oven batteries; CO2 capture, transport, injection and storage infrastructure. (émissions fugitives)
- “GHGs” means greenhouse gases. (GES)
- “GWP” means global warming potential. (PRP)
- “HFCs” means hydrofluorocarbons. (HFC)
- “industrial process emissions” means releases from an industrial process that involves a chemical or physical reaction other than combustion, and the purpose of which is not to produce useful heat or work. This does not include venting from hydrogen production associated with fossil fuel production and processing. (émissions liées aux procédés industriels)
- “industrial product use emissions” means releases from the use of a product for an industrial process that does not involve a chemical or physical reaction and does not react in the process. This includes releases from the use of SF6, HFCs and PFCs as cover gases, and the use of HFCs and PFCs in foam blowing. This does not include releases from PFCs and HFCs in refrigeration, air conditioning, semiconductor manufacturing, fire extinguishing, solvents, aerosols and SF6 in explosion protection, leak detection, electronic applications and fire extinguishing. (émissions associées à l'utilisation de produits industriels)
- “integrated facility” means all buildings, equipment, structures, on-site transportation machinery, and stationary items that are located on a single site, on multiple sites or between multiple sites that are owned or operated by the same person or persons and that function as a single integrated site. “Integrated facility” excludes public roads. (installation intégrée)
- “iron and steel manufacturing” means primary iron and steel production processes, secondary steelmaking processes, iron production processes, coke oven battery production processes, iron ore pellet firing processes, or iron and steel powder processes. (sidérurgie)
- “leakage emissions” means accidental releases and leaks of gases from fossil fuel production and processing, transmission and distribution; iron and steel coke oven batteries; CO2 capture, transport, injection and storage infrastructure for long-term geological storage. (émissions dues aux fuites)
- “lime manufacturing” means all processes that are used to manufacture a lime product by calcination of limestone or other calcareous materials. (fabrication de la chaux)
- “NAICS” means the North American Industry Classification System. (SCIAN)
- “non-variable fuels” means fuels with consistent composition. (combustibles de composition non variable)
- “offshore installation” means an offshore drilling unit, production platform or ship, or sub-sea installation that is attached or anchored to the continental shelf of Canada in connection with the exploitation of oil or natural gas. (installation extracôtière)
- “on-site transportation emissions” means releases from machinery used for the transport or movement of substances, materials, equipment or products that are used in the production process at an integrated facility. This includes releases from vehicles without public road licence. (émissions liées au transport sur le site)
- “PFCs” means perfluorocarbons. (PFC)
- “pipeline transportation system” means all pipelines that are owned or operated by the same person within a province or territory that transport/distribute CO2 or processed natural gas and their associated installations, including meter sets and storage installations but excluding straddle plants or other processing installations. (gazoducs)
- “reporting company” means a person who operates one or more facilities that meet the reporting criteria as set out in Schedule 3 of this notice. (société déclarante)
- “stationary fuel combustion emissions” means releases from stationary fuel combustion sources, in which fuel is burned for the purpose of producing useful heat or work. This includes releases from the combustion of waste fuels to produce useful heat or work. (émissions de combustion stationnaire de combustible)
- “stationary fuel combustion sources” means devices that combust solid, liquid, gaseous, or waste fuel for the purpose of producing useful heat or work . This includes boilers, electricity generating units, cogeneration units, combustion turbines, engines, incinerators, process heaters, and other stationary combustion devices, but does not include emergency flares. (sources de combustion stationnaires)
- “surface leakage” means CO2 emitted from geological formations used for long-term storage of CO2. (fuites en surface)
- “variable fossil fuels” means fuels of variable composition that require the determination of facility specific carbon content. (combustibles fossiles de composition variable)
- “venting emissions” means controlled releases of a process or waste gas, including releases of CO2 associated with carbon capture, transport, injection and storage; from hydrogen production associated with fossil fuel production and processing; of casing gas; of gases associated with a liquid or a solution gas; of treater, stabilizer or dehydrator off-gas; of blanket gases; from pneumatic devices which use natural gas as a driver; from compressor start-ups, pipelines and other blowdowns; from metering and regulation station control loops. (émissions d'évacuation)
- “waste emissions” means releases that result from waste disposal activities at a facility including landfilling of solid waste, flaring of landfill gas, and waste incineration. This does not include releases from the combustion of waste fuels to produce useful heat or work. (émissions des déchets)
- “wastewater emissions” means releases resulting from wastewater and wastewater treatment at a facility. (émissions des eaux usées)
SCHEDULE 3
Reporting criteria
- This notice applies to any person who operates
- (a) a facility that meets a reporting threshold of 10 kt CO2 eq. of the GHG emissions listed in Table 1 of Schedule 1 in the 2017 calendar year;
- (b) a facility that meets a reporting threshold of 10 kt CO2 eq. of the GHG emissions listed in Table 1 of Schedule 1 in the 2017 calendar year is classified under North American Industry Classification System (NAICS) codes 327410, 327310, 331313, or 331110, and is engaged in
- (i) lime manufacturing,
- (ii) cement manufacturing,
- (iii) aluminium manufacturing, or
- (iv) iron and steel manufacturing; or
- (c) a facility engaged in CO2 capture, CO2 transport, CO2 injection or CO2 storage in the 2014, 2015, 2016 or 2017 calendar years.
- Any person who operates a facility described in this notice shall determine whether a facility meets or exceeds the reporting threshold using the following equation:
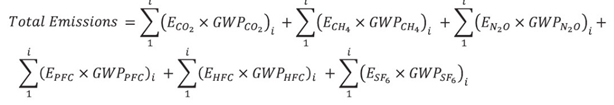
Where:
- E = total emissions of a particular gas or gas species in calendar year 2017, expressed in tonnes
- GWP = global warming potential of the particular gas or gas species, in Table 1 of Schedule 1
- i = each emission source
- (a) determine the quantity of CO2 eq. by multiplying the GWP of a particular GHG or GHG species listed in Table 1 of Schedule 1, by the quantity of a particular GHG or GHG species;
- (b) exclude CO2 emissions from the combustion of biomass in the determination of total emissions;
- (c) exclude CO2 emissions from biomass decomposition in the determination of total emissions.
- Any person who operates a facility that is engaged in more than one activity described in paragraph 1(b) shall report emissions for each activity separately.
- If the person who operates a facility described in paragraphs 1(a) or 1(b) changes during the 2017 calendar year, the facility operator on December 31, 2017, shall report for the entire 2017 calendar year. If facility operations terminate during the 2017 calendar year, the last facility operator shall report for the portion of the year where operations occurred.
- If the person who operates a facility described in paragraph 1(c) changes during the 2014, 2015, 2016 or 2017 calendar years, the facility operator on December 31 shall report for the entire calendar year. If facility operations terminate during the 2014, 2015, 2016 or 2017 calendar years, the last facility operator shall report for the portion of the year where operations occurred.
SCHEDULE 4
Reportable administrative information
- Any person who operates a facility described in Schedule 3 of this notice shall, for each facility, report
- (a) the reporting company's legal and trade name (if any) and federal business number (assigned by the Canada Revenue Agency) and its Dun and Bradstreet (D-U-N-S) number (if any);
- (b) the facility name if any and the address of its physical location;
- (c) the latitude and longitude coordinates of the facility, other than a pipeline transportation system and CO2 transport system;
- (d) the six-digit North American Industry Classification System (NAICS) Canada code;
- (e) the National Pollutant Release Inventory (NPRI) identification number (if any);
- (f) the name, position, mailing and civic address, email address and telephone number of the person submitting the information that is required under this notice;
- (g) the name, position, mailing address, email address and telephone number of the public contact (if any);
- (h) the name, position, mailing and civic address, email address and telephone number of the authorized signing officer signing the Statement of Certification; and
- (i) the legal names of the Canadian parent companies (if any), their civic addresses, their percentage of ownership of the reporting company (where available), their federal business number and their Dun and Bradstreet (D-U-N-S) number if any.
- The reported information required by this notice is to include a Statement of Certification, signed by an authorized signing officer, indicating that the information submitted is true, accurate and complete.
SCHEDULE 5
Reporting requirements
- This schedule applies to any person who operates a facility described in subsection 1 of Schedule 3 of this notice.
- Any person subject to this schedule shall, for each of the GHGs listed in Table 1 of Schedule 1, report
- (a) the total quantity of CO2, CH4 and N2O emissions expressed in tonnes in each of the following source categories: stationary fuel combustion emissions, industrial process emissions, industrial product use emissions, venting emissions, flaring emissions, leakage emissions, on-site transportation emissions, waste emissions and wastewater emissions;
- (b) the total quantity of CH4 and N2O emissions expressed in tonnes from biomass combustion under stationary fuel combustion emissions if the biomass is being burned to produce energy, or under waste emissions in the case of waste incineration and landfill gas flaring processes;
- (c) the total quantity of CO2 emissions expressed in tonnes from biomass combustion; and
- (d) the total quantity of SF6 and each HFC and PFC emissions expressed in tonnes under industrial process emissions and industrial product use emissions.
- Any person subject to this schedule shall
- (a) not account for CO2 emissions from biomass combustion in the total reported facility emissions;
- (b) not report CO2 emissions from biomass decomposition;
- (c) report emissions from coke oven batteries in iron and steel manufacturing under stationary fuel combustion (fuel use for the production of coke), flaring and/or leakage emissions; (see footnote 2) and
- (d) report emissions from hydrogen production in fossil fuel production and processing under venting emissions (see footnote 3) from facilities that are involved in the production, upgrading and refining of fossil fuels.
- Any person subject to this schedule, and to whom any of the Schedules 6 through 11 of this notice apply, shall use the methods described in the applicable schedules to quantify the information that the person must report under this schedule.
- Any person subject to this schedule, and to whom none of the Schedules 6 through 11 of this notice apply, shall
- (a) use methods that are consistent with the 2006 Intergovernmental Panel on Climate Change (IPCC) Guidelines to quantify the information that the person reports under this schedule; and
- (b) report the methods used to determine the quantities reported under paragraphs 2(a), 2(b), 2(c) and 2(d) of this schedule, chosen from monitoring or direct measurement, mass balance, emission factors, or engineering estimates.
| Emission Source Categories | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Greenhouse Gas | Stationary Fuel Combustion Emissions | Industrial Process Emissions | Industrial Product Use Emissions | Fugitive | On-site Transportation Emissions | Waste Emissions | Waste-water Emissions | ||
| Venting Emissions | Flaring Emissions | Leakage Emissions | |||||||
| Carbon dioxide (excluding CO2 emissions from biomass combustion, which is to be reported separately) | N/A | ||||||||
| Methane | N/A | ||||||||
| Nitrous oxide | N/A | ||||||||
| Sulphur hexafluoride | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Hydrofluorocarbons | N/A | by species | by species | N/A | N/A | N/A | N/A | N/A | N/A |
| Perfluorocarbons | N/A | by species | by species | N/A | N/A | N/A | N/A | N/A | N/A |
| Total | |||||||||
SCHEDULE 6
CO2 capture, CO2 transport, CO2 injection and CO2 storage reporting requirements
- This schedule applies to any person who operates a facility described in paragraph 1(c) of Schedule 3 of this notice.
- Any person subject to this schedule shall use the quantification methods for carbon capture, transport and storage described in section 1 of Canada's Greenhouse Gas Quantification Requirements to report the total annual quantity of CO2
- (a) exiting each CO2 capture site, expressed in tonnes (t);
- (b) captured domestically within Canada, entering each CO2 transport system, expressed in tonnes (t);
- (c) imported from outside Canada, entering each CO2 transport system, expressed in tonnes (t);
- (d) exiting each CO2 transport system, expressed in tonnes (t);
- (e) entering each long-term geologic storage site, expressed in tonnes (t);
- (f) injected at each long-term geologic storage site, expressed in tonnes (t);
- (g) entering each enhanced fossil fuel recovery operation, expressed in tonnes (t); and
- (h) injected at each enhanced fossil fuel recovery operation, expressed in tonnes (t).
- Any person subject to this schedule shall use section 1 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the annual weighted average density of mass flow expressed in kilograms per cubic meter (kg/m3), if using the mass flow method;
- (b) the annual weighted average density of volumetric flow with density expressed in kilograms per cubic metre (kg/m3), temperature expressed in degrees Celsius (°C) and pressure expressed in kilopascals (kPa), if using the volumetric flow method;
- (c) the annual weighted average CO2 concentration in the volumetric flow or mass flow, expressed as a mass fraction; and
- (d) the method used to determine the quantities and parameters reported under section 2.
- Any person subject to this schedule shall use section 1 of Canada's Greenhouse Gas Quantification Requirements to report the total annual quantity, expressed in tonnes of CO2 fugitive emissions from equipment and infrastructure used for
- (a) CO2 capture;
- (b) CO2 transport;
- (c) CO2 injection at long-term geological storage site; and
- (d) CO2 injection at enhanced fossil fuel recovery operations.
- Any person subject to this schedule shall report the total annual quantity, expressed in tonnes, of CO2 surface leakage from each long-term geological storage site and enhanced fossil fuel recovery operation.
- Any person subject to this schedule shall use section 1 of Canada's Greenhouse Gas Quantification Requirements to report the total annual quantity, expressed in tonnes of CO2 venting emissions from equipment and infrastructure used for
- (a) CO2 capture;
- (b) CO2 transport;
- (c) CO2 injection at long-term geological storage site; and
- (d) CO2 injection at enhanced fossil fuel recovery operations.
SCHEDULE 7
Fuel combustion reporting requirements
- This schedule applies to any person who operates a facility described in paragraph 1(b) of Schedule 3 of this notice.
- Any person subject to this schedule shall use section 2 of Canada's Greenhouse Gas Quantification Requirements to report the total annual quantity, expressed in tonnes (t), of CO2, CH4 and N2O emissions, by fuel type and source, from
- (a) steam generation, electricity generation, flaring and all other stationary fuel combustion; and
- (b) on-site transportation.
- Any person subject to this schedule shall, for each fuel used under section 2, report
- (a) the gaseous quantities, expressed in cubic metres (m3);
- (b) the solid quantities, expressed in tonnes (t), for coal by rank and by country, province and state; and
- (c) the liquid quantities, expressed in kilolitres (kl).
- Any person subject to this schedule shall, for each fuel used under section 2, report the annual measured and weighted
- (a) high heat value (HHV) following Equation 2-22 in section 2 of Canada's Greenhouse Gas Quantification Requirements, expressed in megajoules (MJ) HHV per unit of fuel consumed for all methods;
- (b) carbon content following Equation 2-23 in section 2 of Canada's Greenhouse Gas Quantification Requirements, expressed in kilograms of carbon per unit of fuel consumed, when using the variable fuels method (except when applying Equation 2-11), or CEMS;
- (c) temperature, expressed in degrees Celsius (°C), and the pressure, expressed in kilopascals (kPa), for gaseous quantities;
- (d) moisture content, expressed as a percentage (%), for solid quantities; and
- (e) CH4 and N2O emission factors, when using the facility specific emission factors measured directly or provided by the fuel supplier or equipment manufacturers, expressed in grams per unit of fuel.
- Any person subject to this schedule shall, for each fuel used under section 2, report the default CO2, CH4 and N2O emission factors when using values presented in Table 2-4 to Table 2-11 of Canada's Greenhouse Gas Quantification Requirements.
- Any person subject to this schedule shall, for steam used to quantify emissions under section 2, report
- (a) the steam quantity expressed in tonnes (t);
- (b) the quantity and type of each biomass fuel combusted expressed in tonnes (t);
- (c) the CO2, CH4 and N2O emission factors, when using the non-variable fuels or variable fuels method, expressed in kilograms of CO2, CH4 and N2O/megajoules (MJ) of steam or kilograms of CO2, CH4 and N2O /tonnes (t) of steam; and
- (d) the measured temperature, expressed in degrees Celsius (°C), the measured pressure expressed in kilopascals (kPa) and the ratio of the boiler's design-rated heat input capacity to its design-rated steam output capacity, expressed in megajoules (MJ)/tonnes of steam, if using the steam default emission factor method.
- Any person subject to this schedule shall report the methods used to quantify each greenhouse gas under section 2 of this schedule, by fuel type and source.
- Any person subject to this schedule shall, for electricity generating units and cogeneration units, report the annual quantities of
- (a) gross electricity generated on-site, expressed in megawatt-hours (MWh);
- (b) electricity sold off-site, expressed in megawatt-hours (MWh);
- (c) electricity lost on-site, expressed in megawatthours (MWh);
- (d) electricity purchased, expressed in megawatthours (MWh);
- (e) gross steam and heat generated on-site, expressed in megajoules (MJ);
- (f) gross steam and heat used to generate electricity on-site, expressed in megajoules (MJ);
- (g) steam and heat sold off-site, expressed in megajoules (MJ);
- (h) steam and heat purchased, expressed in megajoules (MJ); and
- (i) steam or heat lost on-site, expressed in megajoules (MJ).
- Any person subject to this schedule, who
- (a) develops equipment-specific on-site transportation emission factors; or
- (b) quantifies CH4 or N2O emissions using source-specific emission factors determined through measurement or provided by the equipment manufacturer,
- shall submit a document describing the methodology used to develop these factors.
- Any person subject to this schedule, who obtains from a supplier or performs fuel sampling, analysis and consumption measurement, as outlined in section 2.C of Canada's Greenhouse Gas Quantification Requirements, shall submit fuel quantity, carbon content and high heat value for all sampling and measurement periods.
- Any person subject to this schedule is not required to report fuels and their associated emissions when the sum of CO2 emissions from the combustion of one or more of these fuels does not exceed 0.5% of the total facility CO2 emissions from all fuels combusted.
SCHEDULE 8
Lime manufacturing reporting requirements
- This schedule applies to any person who operates a facility described in subparagraph 1(b)(i) of Schedule 3 of this notice.
- Any person subject to this schedule shall use the greenhouse gas quantification methods in section 3 of Canada's Greenhouse Gas Quantification Requirements to report on an annual basis
- (a) the total annual quantity of CO2 emissions from lime production, expressed in tonnes (t);
- (b) the total monthly quantity of lime, by lime type, expressed in tonnes (t);
- (c) the monthly plant specific emission factor, by lime type, expressed in tonnes of CO2/tonnes of lime;
- (d) the monthly calcium oxide (CaO) content of lime, by lime type, expressed in tonnes of CaO/tonnes of lime;
- (e) the monthly magnesium oxide (MgO) content of lime, by lime type, expressed in tonnes of MgO/tonnes of lime;
- (f) the total annual quantity of CO2 emissions from calcined by-products/wastes, by by-product/waste type, expressed in tonnes (t);
- (g) the total quarterly quantity of calcined by-products/wastes, by by-product/waste type, expressed in tonnes (t);
- (h) the quarterly plant-specific emission factor of calcined by-products/wastes, by calcined by-product/waste type, expressed in tonnes of CO2/tonnes of by-product/waste;
- (i) the quarterly weighted average calcium oxide (CaO) content of calcined by-products/wastes, by calcined by-product/waste type, expressed in tonnes of CaO/tonnes of by-product/waste; and
- (j) the quarterly weighted average magnesium oxide (MgO) content of calcined by-products/wastes, by calcined by-product/waste type, expressed in tonnes of MgO/tonnes of by-product/waste.
- Any person described in this schedule who operates a facility with stack(s) monitored by CEMS may use the annual emissions data from CEMS to report the emissions and production quantities under paragraphs 2(a), (b), (f) and (g). This shall not include emissions information specified for CEMS in Schedule 7 of this notice. The person shall indicate where CEMS is being used to calculate emissions.
SCHEDULE 9
Cement manufacturing reporting requirements
- This schedule applies to any person who operates a facility described in subparagraph 1(b)(ii) of Schedule 3 of this notice.
- Any person subject to this schedule shall use the greenhouse gas quantification methods in section 4 of Canada's Greenhouse Gas Quantification Requirements to report on an annual basis
- (a) the total annual quantity of CO2 emissions from clinker production, expressed in tonnes (t);
- (b) the total monthly quantity of clinker, expressed in tonnes (t);
- (c) the monthly plant-specific emission factor of clinker, expressed in tonnes of CO2/tonnes of clinker;
- (d) the monthly calcium oxide (CaO) content of clinker, expressed in tonnes of CaO/tonnes of clinker;
- (e) the monthly magnesium oxide (MgO) content of clinker, expressed in tonnes of MgO/tonnes of clinker;
- (f) the monthly non-calcined calcium oxide (CaO) content of clinker, expressed in tonnes of CaO/tonnes of clinker;
- (g) the monthly non-calcined magnesium oxide (MgO) content of clinker, expressed in tonnes of MgO/tonnes of clinker;
- (h) the monthly quantity of non-carbonate raw materials entering the kiln, expressed in tonnes (t);
- (i) the total annual quantity of CO2 emissions from organic carbon oxidation, expressed in tonnes (t);
- (j) the total annual quantity of raw material consumption, expressed in tonnes (t);
- (k) the annual weighted average carbon content in raw material consumption, expressed in tonnes of C/tonnes of raw material consumption;
- (l) the total annual quantity of CO2 emissions from cement kiln dust (CKD) not recycled back to the kiln, expressed in tonnes (t);
- (m) the total quarterly quantity of CKD not recycled back to the kiln, expressed in tonnes (t);
- (n) the quarterly plant specific emission factor of CKD not recycled back to the kiln, expressed in tonnes of CO2/tonnes of CKD;
- (o) the quarterly calcium oxide (CaO) content of CKD not recycled back to the kiln, expressed in tonnes of CaO/tonnes of CKD;
- (p) the quarterly magnesium oxide (MgO) content of CKD not recycled back to the kiln, expressed in tonnes of MgO/tonnes of CKD;
- (q) the quarterly non-calcined calcium oxide (CaO) content of CKD not recycled back to the kiln, expressed in tonnes of CaO/tonnes of CKD; and
- (r) the quarterly non-calcined magnesium oxide (MgO) content of CKD not recycled back to the kiln, expressed in tonnes of MgO/tonnes of CKD.
- Any person subject to this schedule who operates a facility with stack(s) monitored by CEMS may use the annual emissions data from CEMS to report the emissions and production information under paragraphs 2(a), (b), (h), (i), (l) and (m). This shall not include the emissions information specified for CEMS in Schedule 7 of this notice. The person shall indicate where CEMS is being used to calculate emissions.
SCHEDULE 10
Aluminium manufacturing reporting requirements
- This schedule applies to any person who operates a facility described in subparagraph 1(b)(iii) of Schedule 3 of this notice.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.1 of Canada's Greenhouse Gas Quantification Requirements to report on an annual basis
- (a) the total annual quantity of CO2 emissions from prebaked anode consumption, expressed in tonnes (t);
- (b) the monthly anode consumption, expressed in tonnes of anodes/tonnes of liquid aluminium production;
- (c) the monthly sulphur content of prebaked anodes, expressed in kilograms of S/kilograms of prebaked anodes; and
- (d) the monthly ash content of prebaked anodes, expressed in kilograms of ash/kilograms of prebaked anodes.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.2 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the total annual quantity of CO2 emissions from anode consumption from Søderberg cells, expressed in tonnes (t);
- (b) the total monthly quantity of CSM emissions, expressed in tonnes, or the International Aluminium Institute factor used, expressed in kilograms of CSM/tonnes of liquid aluminium;
- (c) the total monthly anode paste consumption, expressed in tonnes of paste/tonnes of liquid aluminium;
- (d) the monthly average content of pitch or other binding agent in paste, expressed in kilograms of pitch or other binding agent/kilograms of paste;
- (e) the monthly sulphur content in pitch or other binding agent, expressed in kilograms of S/kilograms of pitch or other binding agent;
- (f) the monthly ash content in pitch or other binding agent, expressed in kilograms of ash/kilograms of pitch or other binding agent;
- (g) the monthly hydrogen content in pitch or other binding agent, expressed in kilograms of H2/kilograms of pitch or other binding agent or the International Aluminium Institute factor used;
- (h) the monthly sulphur content in calcinated coke, expressed in kilograms of S/kilograms of calcinated coke;
- (i) the monthly ash content in calcinated coke, expressed in kilograms of ash/kilograms of calcinated coke; and
- (j) the monthly carbon content in dust from Søderberg electrolysis cells, expressed in kilograms of C/kilograms of liquid aluminium, or a value of 0.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.3 of Canada's Greenhouse Gas Quantification Requirements to report the total annual quantity of CO2 emissions from anode and cathode baking, expressed in tonnes (t).
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.4 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the total annual quantity of CO2 emissions from packing material consumption, expressed in tonnes (t);
- (b) the annual packing material consumption, expressed in tonnes of packing of material/tonnes of baked anodes or cathodes;
- (c) the total annual quantity of baked anodes and cathodes removed from the furnace, expressed in tonnes (t);
- (d) the annual weighted average ash content of packing material, expressed in kilograms of ash/kilograms of packing material; and
- (e) the annual weighted average sulphur content of packing material, expressed in kilograms of S/kilograms of packing material.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.5 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the total annual quantity of CO2 emissions from coking of pitch or other binding agent, expressed in tonnes (t);
- (b) the total annual quantity of green anodes or cathodes put into the furnace, expressed in tonnes (t);
- (c) the total annual quantity of baked anodes or cathodes removed from the furnace, expressed in tonnes (t);
- (d) the annual weighted average hydrogen content of pitch or other binding agent or the International Aluminium Institute factor used, expressed in kilograms of H2/kilograms of pitch or other binding agent;
- (e) the annual weighted average pitch content of green anodes or cathodes, expressed in kilograms of pitch or other binding agent/kilograms of anodes or cathodes; and
- (f) the total annual quantity of recovered tar, expressed in tonnes (t).
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.6 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the total annual quantity of CO2 emissions from green coke calcination, expressed in tonnes (t);
- (b) the total monthly quantity of CO2 emissions from coke dust, expressed in tonnes (t);
- (c) the total monthly quantity of green coke consumption, expressed in tonnes (t);
- (d) the total monthly quantity of calcinated coke production, expressed in tonnes (t);
- (e) the total monthly quantity of under-calcinated coke production, expressed in tonnes (t);
- (f) the monthly water content in green coke, expressed in kilograms of H2O/kilograms of green coke;
- (g) the monthly volatile materials content in green coke, expressed in kilograms of volatile materials/ kilograms of green coke;
- (h) the monthly sulphur content in green coke, expressed in kilograms of S/kilograms of green coke; and
- (i) the monthly sulphur content in calcinated coke, expressed in kilograms of S/kilograms of calcinated coke.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.7 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the total annual quantity of CF4 emissions from anode effects, expressed in tonnes (t);
- (b) the monthly slope, if using the slope method, by a series of pots, expressed in tonnes of CF4/tonnes of liquid aluminium/anode effect minute/pot-day/year;
- (c) the monthly anode effect duration, if using the slope method, expressed in anode effect minutes/pot-day calculated per year and obtained by multiplying the anode effects frequency, in number of anode effects per pot-day, by the average duration of anode effects in minutes;
- (d) the overvoltage coefficient, if using the overvoltage coefficient method, expressed in tonnes of CF4/tonnes of liquid aluminium/millivolt;
- (e) the monthly anode effect overvoltages, if using the overvoltage coefficient method, expressed in millivolts/pot;
- (f) the current efficiency of the aluminium production process, if using the overvoltage coefficient method, expressed as a fraction; and
- (g) the method used to determine the quantities reported under paragraph (a).
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.7 of Canada's Greenhouse Gas Quantification Requirements to report
- (a) the total annual quantity of C2F6 emissions, expressed in tonnes (t); and
- (b) the weight fraction of C2F6 to CF4 or selected from Table 5-2, expressed in kilograms of C2F6/kilograms of CF4.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 5.A.8 of Canada's Greenhouse Gas Quantification Requirements to report the total annual quantity of emissions from SF6 used as a cover gas, expressed in tonnes (t).
- Any person subject to this schedule shall report the total annual quantity of liquid aluminium production, expressed in tonnes (t).
- Any person subject to this schedule who operates a facility with stack(s) monitored by CEMS may use the annual emissions data from CEMS to report the emissions under sections 2 to 7 of this schedule. This shall not include the emissions information specified for CEMS in Schedule 7 of this notice. The person shall indicate where CEMS is being used to calculate emissions.
SCHEDULE 11
Iron and steel manufacturing reporting requirements
- This schedule applies to any person who operates a facility described in subparagraph 1(b)(iv) of Schedule 3 of this notice.
- Any person subject to this schedule shall report on an annual basis
- (a) the total annual quantity of biomass consumed, by biomass type, expressed in tonnes (t); and
- (b) the type of use for biomass (such as flux material, reducing agent).
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.1 of Canada's Greenhouse Gas Quantification Requirements for a taconite indurating furnace to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of greenball/taconite pellets consumption, expressed in tonnes, if using equation 6-1;
- (c) the annual weighted average carbon content of greenball/taconite pellets consumption, expressed in tonnes of C/tonnes of greenball/taconite pellets, if using equation 6-1;
- (d) the total annual quantity of additive material consumption, by material type, expressed in tonnes, if using equation 6-2;
- (e) the annual weighted average carbon content of additive material consumption, expressed in tonnes of C/tonnes of additive material, if using equation 6-2;
- (f) the total annual quantity of iron ore pellets fed to the furnace, expressed in tonnes, if using equation 6-2;
- (g) the annual weighted average carbon content of iron ore pellets fed to the furnace, expressed in tonnes of C/tonnes iron ore pellets;
- (h) the total annual quantity of fired pellet production, expressed in tonnes (t);
- (i) the annual weighted average carbon content of fired pellet production, expressed in tonnes of C/tonnes of fired pellets;
- (j) the annual quantity of air pollution control residue collected, expressed in tonnes (t);
- (k) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes residue; and
- (l) the method used to determine the quantities under paragraph (a) above.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.2 of Canada's Greenhouse Gas Quantification Requirements for a basic oxygen furnace to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of molten iron charged to the furnace, expressed in tonnes (t);
- (c) the annual weighted average carbon content of molten iron charged to the furnace, expressed in tonnes of C/tonnes of molten iron;
- (d) the total annual quantity of ferrous scrap charged to the furnace, expressed in tonnes (t);
- (e) the annual weighted average carbon content of ferrous scrap charged to the furnace, expressed in tonnes of C/tonnes of ferrous scrap;
- (f) the total annual quantity of carbonaceous material consumption, by material type, expressed in tonnes (t);
- (g) the annual weighted average carbon content of non-biomass carbonaceous material consumption, by material type, expressed in tonnes of C/tonnes of carbonaceous material;
- (h) the total annual quantity of flux material charged to the furnace, by material type, expressed in tonnes (t);
- (i) the annual weighted average carbon content of non-biomass flux material charged to the furnace, expressed in tonnes of C/tonnes of flux;
- (j) the total annual quantity of molten raw steel production, expressed in tonnes (t);
- (k) the annual weighted average carbon content of molten raw steel production, expressed in tonnes of C/tonnes of molten raw steel;
- (l) the total annual quantity of slag production, expressed in tonnes (t);
- (m) the annual weighted average carbon content of slag production, expressed in tonnes of C/tonnes of slag;
- (n) the total annual quantity of furnace gas transferred off-site, expressed in tonnes (t);
- (o) the annual weighted average carbon content of furnace gas transferred off site, expressed in tonnes of C/tonnes of furnace gas transferred;
- (p) the total annual quantity of air pollution control residue collected, expressed in tonnes (t); and
- (q) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.3 of Canada's Greenhouse Gas Quantification Requirements for coke oven battery to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of coking coal charged to battery, expressed in tonnes (t);
- (c) the annual weighted average carbon content of non-biomass coking coal charged to battery, expressed in tonnes of C/tonnes of coking coal;
- (d) the total annual quantity of coke produced, expressed in tonnes (t);
- (e) the annual weighted average carbon content of coke produced, expressed in tonnes of C/tonnes of coke;
- (f) the total annual quantity of coke oven gas transferred off site, expressed in tonnes (t);
- (g) the annual weighted average carbon content of coke oven gas transferred off site, expressed in tonnes of C/tonnes of coke oven gas;
- (h) the total annual quantity of by-product from coke oven battery, expressed in tonnes (t);
- (i) the annual weighted average carbon content of non-biomass by-product from coke oven battery, expressed in tonnes of C/tonnes of by-product;
- (j) the total annual quantity of air pollution control residue collected, expressed in tonnes (t); and
- (k) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.4 of Canada's Greenhouse Gas Quantification Requirements for sinter production to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of carbonaceous material consumption, by material type, expressed in tonnes (t);
- (c) the annual weighted average carbon content of non-biomass carbonaceous material consumption, by material type, expressed in tonnes of C/tonnes of carbonaceous material;
- (d) the total annual quantity of sinter feed material, expressed in tonnes (t);
- (e) the annual weighted average carbon content of sinter feed material , expressed in tonnes of C/tonnes sinter feed;
- (f) the total annual quantity of sinter production, expressed in tonnes (t);
- (g) the annual weighted average carbon content of sinter production, expressed in tonnes of C/tonnes sinter production;
- (h) the total annual quantity air pollution control residue collected, expressed in tonnes (t); and
- (i) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.5 of Canada's Greenhouse Gas Quantification Requirements for an electric arc furnace to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of direct reduced iron charged to the furnace, expressed in tonnes (t);
- (c) the annual weighted average carbon content of direct reduced iron charged to the furnace, expressed in tonnes of C/tonnes of direct reduced iron;
- (d) the total annual quantity of ferrous scrap charged to the furnace, expressed in tonnes (t);
- (e) the annual weighted average carbon content of ferrous scrap charged to the furnace, expressed in tonnes of C/tonnes of ferrous scrap;
- (f) the total annual quantity of carbonaceous material consumption, by material type, expressed in tonnes (t);
- (g) the annual weighted average carbon content of non-biomass carbonaceous material consumption, by material type, expressed in tonnes of C/tonnes of carbonaceous material;
- (h) the total annual quantity of carbon electrode consumption, expressed in tonnes (t);
- (i) the annual weighted average carbon content of non-biomass carbon electrode consumption, expressed in tonnes of C/tonnes of carbon electrode;
- (j) the total annual quantity of flux material charged to the furnace, by material type, expressed in tonnes (t);
- (k) the annual weighted average carbon content of non-biomass flux material charged to the furnace, expressed in tonnes of C/tonnes of flux;
- (l) the total annual quantity of molten raw steel production, expressed in tonnes (t);
- (m) the annual weighted average carbon content of molten raw steel production, expressed in tonnes of C/tonnes of molten raw steel;
- (n) the total annual quantity of slag production, expressed in tonnes (t);
- (o) the annual weighted average carbon content of slag production, expressed in tonnes of C/tonnes of slag;
- (p) the total annual quantity air pollution control residue collected, expressed in tonnes (t); and
- (q) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.6 of Canada's Greenhouse Gas Quantification Requirements for an argon-oxygen decarburization vessel to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of molten steel charged to the vessel, expressed in tonnes (t);
- (c) the annual weighted average carbon content of molten steel charged to the vessel, expressed in tonnes of C/tonnes of molten raw steel;
- (d) the annual weighted average carbon content of molten steel before decarburization, expressed in tonnes of C/tonnes of molten steel;
- (e) the annual weighted average carbon content of molten steel after decarburization, expressed in tonnes of C/tonnes of molten steel;
- (f) the total annual quantity of air pollution control residue collected, expressed in tonnes (t); and
- (g) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.7 of Canada's Greenhouse Gas Quantification Requirements for a direct reduction furnace to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of iron ore or iron ore pellets consumption, expressed in tonnes (t);
- (c) the annual weighted average carbon content of iron ore or iron ore pellets consumption, expressed in tonnes of C/tonnes of iron ore or iron ore pellets;
- (d) the total annual quantity of consumed raw material other than carbonaceous material and ore, by material type, expressed in tonnes (t);
- (e) the annual weighted average carbon content of raw material, other than carbonaceous material and ore, by material type expressed in tonnes of C/tonnes of raw material;
- (f) the total annual quantity of carbonaceous material consumption, by material type, expressed in tonnes (t);
- (g) the annual weighted average carbon content of non-biomass carbonaceous material consumption, by material type, expressed in tonnes of C/tonnes of carbonaceous material;
- (h) the total annual quantity of iron production, expressed in tonnes (t);
- (i) the annual weighted average carbon content of iron production, expressed in tonnes of C/tonnes of iron;
- (j) the total annual quantity of non-metallic material production, expressed in tonnes (t);
- (k) the annual weighted average carbon content of non-metallic material production, expressed in tonnes of C/tonnes of non-metallic material;
- (l) the total annual quantity of air pollution control residue collected, expressed in tonnes (t); and
- (m) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.8 of Canada's Greenhouse Gas Quantification Requirements for a blast furnace to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of iron ore or iron ore pellets consumption, expressed in tonnes (t);
- (c) the annual weighted average carbon content of iron ore or iron ore pellets consumption, expressed in tonnes of C/tonnes of iron ore or iron ore pellets;
- (d) the total annual quantity of consumed raw material other than carbonaceous material and ore, by material type, expressed in tonnes (t);
- (e) the annual average carbon content of raw material, other than carbonaceous material and ore, by material type, expressed in tonnes of C/tonnes of raw material;
- (f) the total annual quantity of carbonaceous material consumption, by material type, expressed in tonnes (t);
- (g) the annual weighted average carbon content of non-biomass carbonaceous material consumption, by material type, expressed in tonnes of C/tonnes of carbonaceous material;
- (h) the total annual quantity of flux material charged to the furnace, by material type, expressed in tonnes (t);
- (i) the annual weighted average carbon content of non-biomass flux material charged to the furnace, expressed in tonnes of C/tonnes of flux;
- (j) the total annual quantity of iron production, expressed in tonnes (t);
- (k) the annual weighted average carbon content of iron production, expressed in tonnes of C/tonnes of iron;
- (l) the total annual quantity of non-metallic material production, expressed in tonnes (t);
- (m) the annual weighted average carbon content of non-metallic material production, expressed in tonnes of C/tonnes of non-metallic material;
- (n) the total annual quantity of blast furnace gas transferred off-site, expressed in tonnes (t);
- (o) the annual weighted average carbon content of blast furnace gas transferred off-site, expressed in tonnes of C/tonnes blast furnace gas;
- (p) the total annual quantity of air pollution control residue collected, expressed in tonnes (t); and
- (q) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.A.9 of Canada's Greenhouse Gas Quantification Requirements for the ladle furnace to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of molten steel fed to the furnace, expressed in tonnes (t);
- (c) the annual weighted average carbon content of molten steel fed to the furnace, expressed in tonnes of C/tonnes of molten steel;
- (d) the total annual quantity of additive material consumed by the furnace, by material type, expressed in tonnes (t);
- (e) the annual weighted average carbon content of additive material consumed by the furnace, by material type, expressed in tonnes of C/tonnes of additive material;
- (f) the total annual carbon electrodes consumed by the furnace, expressed in tonnes (t);
- (g) the annual weighted average carbon content of carbon electrodes consumed by the furnace, expressed in tonnes of C/tonnes of carbon electrodes;
- (h) the total annual quantity of molten steel production, expressed in tonnes (t);
- (i) the annual weighted average carbon content of molten steel production, expressed in tonnes of C/tonnes of molten steel;
- (j) the total annual quantity of slag production, expressed in tonnes (t);
- (k) the annual weighted average carbon content of slag production, or a default value of 0, expressed in tonnes of C/tonnes of slag;
- (l) the total annual quantity of air pollution control residue collected, expressed in tonnes (t);
- (m) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue;
- (n) the total annual quantity of other residue produced, expressed in tonnes (t); and
- (o) the annual weighted average carbon content of other residue produced or a default value of 0, expressed in tonnes of C/tonnes of residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.B.1 of Canada's Greenhouse Gas Quantification Requirements for the atomization of molten cast iron to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of molten cast iron fed into the process, expressed in tonnes (t);
- (c) the annual weighted average carbon content of molten cast iron fed into the process, expressed in tonnes of C/tonnes of molten cast iron;
- (d) the total annual quantity of other material used in process, by material type, expressed in tonnes (t);
- (e) the annual weighted average carbon content of other material used in process, by material type, expressed in tonnes of C/tonnes of other material;
- (f) the total annual quantity of atomized cast iron production, expressed in tonnes (t);
- (g) the annual weighted average carbon content of atomized cast iron production, expressed in tonnes of C/tonnes of atomized cast iron;
- (h) the total annual quantity of by-products, by by-product type, expressed in tonnes (t); and
- (i) the annual weighted average carbon content of by-products, by by-product type, expressed in tonnes of C/tonnes of by-product.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.B.2 of Canada's Greenhouse Gas Quantification Requirements for the decarburization of iron powder to report
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of iron powder fed into the process, expressed in tonnes (t);
- (c) the annual weighted average carbon content of iron powder fed into the process, expressed in tonnes of C/tonnes of iron powder;
- (d) the total annual quantity of decarburized iron powder production, expressed in tonnes (t);
- (e) the annual weighted average carbon content of decarburized iron powder production, expressed in tonnes of C/tonnes of decarburized iron powder production;
- (f) the total annual quantity of by-product, by by-product type, expressed in tonnes (t); and
- (g) the annual weighted average carbon content of by-product, by by-product type, expressed in tonnes of C/tonnes of by-product.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.B.3 of Canada's Greenhouse Gas Quantification Requirements for steel grading to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of molten steel fed into the process, expressed in tonnes (t);
- (c) the annual weighted average carbon content of molten steel fed into the process, expressed in tonnes of C/tonnes of molten steel;
- (d) the total annual quantity of additive used in the process, expressed in tonnes (t);
- (e) the annual weighted average carbon content of additive used in the process, by additive type, expressed in tonnes of C/tonnes of additive;
- (f) the total annual quantity of carbon electrode consumption, expressed in tonnes (t);
- (g) the annual weighted average carbon content of carbon electrode consumption, expressed in tonnes of C/tonnes of carbon electrode consumption;
- (h) the total annual quantity of molten steel production, expressed in tonnes (t);
- (i) the annual weighted average carbon content of molten steel production, expressed in tonnes of C/tonnes of molten steel production;
- (j) the total annual quantity of slag production, expressed in tonnes (t);
- (k) the annual weighted average carbon content of slag production, expressed in tonnes of C/tonnes of slag production;
- (l) the total annual quantity of air pollution control residue collected, expressed in tonnes (t);
- (m) the annual weighted average carbon content of air pollution control residue collected, expressed in tonnes of C/tonnes of residue;
- (n) the total annual quantity of other residue production, expressed in tonnes (t); and
- (o) the annual weighted average carbon content of other residue production, expressed in tonnes of C/tonnes of other residue.
- Any person subject to this schedule shall use the greenhouse gas quantification method(s) in section 6.B.4 of Canada's Greenhouse Gas Quantification Requirements for steel powder annealing to report on an annual basis
- (a) the total annual quantity of CO2 emissions, expressed in tonnes (t);
- (b) the total annual quantity of steel powder fed into the process, expressed in tonnes (t);
- (c) the annual weighted average carbon content of steel powder fed into the process, expressed in tonnes of C/tonnes of steel powder;
- (d) the total annual quantity of steel powder production, expressed in tonnes (t);
- (e) the annual weighted average carbon content of steel powder production, expressed in tonnes of C/tonnes of steel powder production;
- (f) the total annual quantity of by-product, by by-product type, expressed in tonnes (t); and
- (g) the annual weighted average carbon content of by-product, by by-product type, expressed in tonnes of C/tonnes of by-product.
- Any person subject to this schedule who operates a facility with stack(s) monitored by CEMS shall use the greenhouse gas quantification method(s) in section 6.A of Canada's Greenhouse Gas Quantification Requirements for iron and steel production to report
- (a) the CO2 emissions information under sections 3 to 15 of this schedule separately from CO2 emissions information specified for CEMS in Schedule 7 of this notice; and
- (b) the production information specified under paragraphs 3(h), 4(j), 5(d), 6(f), 7(l), 7(n), 8(b), 9(h), 9(j), 10(j), 10(l), 11(h), 11(j), 12(f), 13(d), 14(h), 14(j) and 15(d).
The person shall indicate where CEMS is being used to calculate emissions.
EXPLANATORY NOTE
(This note is not part of the notice.)
In March of 2004, the Government of Canada established the Greenhouse Gas Reporting Program (GHGRP) to collect greenhouse gas (GHG) emissions information annually from the largest emitting Canadian facilities. Under this mandatory reporting program, a notice is issued in accordance with section 46 of the Act and published annually in the Canada Gazette, outlining the reporting requirements. Operators of facilities that meet the criteria specified in the notice are required to submit their information to Environment and Climate Change Canada by June 1 of each year. The GHGRP is part of Canada's ongoing effort to develop, through a collaborative process with provinces and territories, a harmonized and efficient reporting system that will meet the information needs of all levels of government, provide Canadians with reliable and timely information on greenhouse gas emissions and support regulatory initiatives.
In December 2016, the Government of Canada published the Notice of intent to inform stakeholders of upcoming consultations on proposed changes to the Greenhouse Gas Reporting Program. It is pursuing this expansion to the GHGRP in order to
- enable direct use of the reported data in Canada's National Greenhouse Gas Inventory;
- increase the consistency and comparability of GHG data across jurisdictions; and
- obtain a more comprehensive picture of emissions by Canadian facilities.
A proposed set of expanded reporting requirements and methods, applicable to 2017 calendar year, was circulated and consultations took place throughout 2017.
This notice represents the first year of the phased expansion to the GHG reporting requirements for industrial facilities in Canada. It contains the following key changes:
- The reporting threshold has been lowered from 50 kt to 10 kt. All facilities emitting the equivalent of 10 kt or more of GHGs in carbon dioxide equivalent (CO2 eq.) units in 2017 are required to submit a report.
- Requirements to provide additional data related to GHG emissions and apply specific quantification methods to determine emissions. These requirements are specific to manufacturers of cement, lime, iron and steel, and aluminium, as well as to facilities engaged in carbon capture, transport and storage activities.
Information required to be reported as outlined in this notice will continue to be collected via Environment and Climate Change Canada's (ECCC) Single Window (SW) system. This system currently collects data for ECCC's GHGRP and for British Columbia, Alberta, Ontario and New Brunswick, to support provincial GHG reporting regulations; the National Pollutant Release Inventory and its partners and various other partner programs. Further information on the GHGRP and step-by-step instructions on how to navigate the SW system are available on the Program website: https://www.canada.ca/en/environment-climate-change/services/climate-change/greenhouse-gas-emissions/facility-reporting/reporting.html.
Compliance with the Act is mandatory and specific offences are established by subsections 272(1), 272.1(1) 272.2(1) 272.4(1) and 272.5(1) of the Act. Subsections 272(2), (3) and (4) and 272.1(2), (3) and (4) of the Act set the penalties for persons who commit an offence under the Act. Offences include the offence of failing to comply with an obligation arising from the Act and the offence of providing false or misleading information. Penalties for the most serious offences include minimum fines and/or imprisonment. The amount of the fine can range from a minimum of $5,000 for an individual convicted following summary proceedings and/or to imprisonment for a term of up to 6 months, to a maximum of $6,000,000 for a large corporation convicted on indictment. The fine range doubles for second or subsequent offences and individuals may also be liable to a term of imprisonment of up to three years. Offences other than those in the category of “serious offences” are punishable by fines set at a maximum that ranges from $25,000 for an individual convicted following summary proceedings to $500,000 for a large corporation convicted on indictment. The maximum fines are double for second or subsequent offences.
The current text of the Act, including the most recent amendments, is available on Justice Canada's website: laws-lois.justice.gc.ca/eng/acts/C-15.31/.
The Act is enforced in accordance with the Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999 available at https://www.canada.ca/en/environment-climate-change/services/canadian-environmental-protection-act-registry/publications/compliance-enforcement-policy.html. Suspected violations under the Act can be reported to the Enforcement Branch by email at ec.enviroinfo.ec@canada.ca.
An electronic copy of this notice is available at the following Internet addresses: ec.gc.ca/lcpe-cepa/eng/notices/default.cfm or https://www.canada.ca/en/environment-climate-change/services/climate-change/greenhouse-gas-emissions.html.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
DEPARTMENT OF HEALTH
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Publication after screening assessment of four carboxylic acids specified on the Domestic Substances List (subsection 77(1) of the Canadian Environmental Protection Act, 1999)
Whereas the four substances identified in the annex below are substances identified under subsection 73(1) of the Canadian Environmental Protection Act, 1999;
Whereas a summary of the draft screening assessment conducted on these substances pursuant to section 74 of the Act is annexed hereby;
And whereas it is proposed to conclude that the substances do not meet any of the criteria set out in section 64 of the Act,
Notice therefore is hereby given that the Minister of the Environment and the Minister of Health (the ministers) propose to take no further action on these substances at this time under section 77 of the Act.
Public comment period
As specified under subsection 77(5) of the Canadian Environmental Protection Act, 1999, any person may, within 60 days after publication of this notice, file with the Minister of the Environment written comments on the measure the ministers propose to take and on the scientific considerations on the basis of which the measure is proposed. More information regarding the scientific considerations may be obtained from the Canada.ca (Chemical Substances) website. All comments must cite the Canada Gazette, Part I, and the date of publication of this notice and be sent to the Executive Director, Program Development and Engagement Division, Department of the Environment, Gatineau, Quebec K1A 0H3, by fax to 819-938-5212, or by email to eccc.substances.eccc@canada.ca.
In accordance with section 313 of the Canadian Environmental Protection Act, 1999, any person who provides information in response to this notice may submit with the information a request that it be treated as confidential.
Jacqueline Gonçalves
Director General
Science and Risk Assessment Directorate
On behalf of the Minister of the Environment
David Morin
Director General
Safe Environments Directorate
On behalf of the Minister of Health
ANNEX
Summary of the draft screening assessment of the Carboxylic Acids Group
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively as the Carboxylic Acids Group. Substances in this group were identified as priorities for assessment, as they met the categorization criteria under subsection 73(1) of CEPA. Their Chemical Abstracts Service Registry Number (see footnote 4) (CAS RN), their Domestic Substances List (DSL) name and their common name are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
79-09-4 |
Propanoic acid |
Propionic acid |
107-92-6 |
Butanoic acid |
n-Butyric acid |
112-05-0 |
Nonanoic acid |
Nonanoic acid |
144-62-7 |
Ethanedioic acid |
Oxalic acid |
In 2011, imported quantities of n-butyric acid and oxalic acid were reported to range from 10 000 to 100 000 kg, imported quantities of nonanoic acid were reported to be 28 925 kg and imported quantities of propionic acid were reported to range from 1 000 000 to 10 000 000 kg. None of these substances were reported to be manufactured in Canada in 2011 above the reporting threshold of 100 kg.
The substances in the Carboxylic Acids Group are reported to be used commercially in Canada in a number of applications such as processing aids, plastic and rubber materials, industrial intermediates, lubricants, solvents, and non-pesticidal agricultural products.
These substances are naturally occurring compounds. Propionic and n-butyric acids are endogenous to humans, as they are produced through microbial fermentation in the gastrointestinal tract. Propionic acid, n-butyric acid, and nonanoic acid occur naturally in a variety of foods and may also be used as food flavouring agents. In Canada, propionic acid is also an approved food additive. Propionic acid and oxalic acid are used as components in the manufacture of a variety of food packaging materials. Nonanoic and oxalic acids are used as components in incidental additives for use in food processing establishments. Oxalic acid is also a naturally occurring substance in some foods and has been identified as an ingredient in cleaning products available to consumers in Canada.
All of the substances in the Carboxylic Acids Group are registered pesticide formulants in Canada. They are also all permitted ingredients in natural health products, and propionic, n-butyric, and oxalic acids have been identified in natural health products. Some of these substances are also present in products available to consumers, such as nonanoic acid in eye make-up and cleaning products, and oxalic acid in cleaning products.
The ecological risks of substances in the Carboxylic Acids Group in this draft screening assessment were characterized using the Ecological Risk Classification (ERC) of organic substances. The ERC is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established based principally on metrics regarding mode of toxic action, chemical reactivity, food web–derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. The ERC identified propionic acid, n-butyric acid, nonanoic acid, and oxalic acid as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is a low risk of harm to organisms and the broader integrity of the environment from propionic acid, n-butyric acid, nonanoic acid, and oxalic acid. It is proposed to conclude that propionic acid, n-butyric acid, nonanoic acid, and oxalic acid do not meet the criteria under paragraph 64(a) or (b) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, propionic acid has been assessed by the Organisation for Economic Co-operation and Development (OECD) in 2007 and by the European Food Safety Authority (EFSA) in 2014; n-butyric acid was assessed by OECD in 2003, and nonanoic acid has been assessed by the European Chemicals Agency (ECHA 2013). Potential sources of exposure of the general population to propionic and n-butyric acids would be from their natural occurrence in the environment and in foods, their uses as food additives or flavouring agents, as well as their uses in natural health products and/or homeopathic products. Potential sources of exposure of the general population to nonanoic acid include its natural occurrence in the environment and in foods, its uses as a food flavouring substance, and its uses as an ingredient in an eye make-up product and as an ingredient in a liquid disinfectant solution. Based on information from the above-noted international assessments, propionic acid, n-butyric acid, and nonanoic acid are considered to be substances of low hazard potential; therefore, risk to human health is considered to be low.
Exposure to oxalic acid can occur from its use as an ingredient in cleaning products and its natural presence in foods. The available health effects information on oxalic acid indicates potential effects on the reproductive system. The margins of exposure between estimated exposures of oxalic acid and the critical effect level in laboratory studies are considered adequate to address uncertainties in the health effects and exposure databases.
Based on the information presented in this draft screening assessment, it is proposed to conclude that propionic acid, n-butyric acid, nonanoic acid, and oxalic acid do not meet the criteria under paragraph 64(c) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Proposed conclusion
It is proposed to conclude that propionic acid, n-butyric acid, nonanoic acid, and oxalic acid do not meet any of the criteria set out in section 64 of CEPA.
The draft screening assessment for these substances is available on the Canada.ca (Chemical Substances) website.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Significant New Activity Notice No. 19179
Significant New Activity Notice
(Section 85 of the Canadian Environmental Protection Act, 1999)
Whereas the Minister of the Environment and the Minister of Health have assessed information in respect of the substance quaternary ammonium compounds, benzylalkyldimethyl, salts with bentonite, Confidential Accession No. 19214-2, under section 83 of the Canadian Environmental Protection Act, 1999;
Whereas the substance is not specified on the Domestic Substances List;
And whereas the ministers suspect that a significant new activity in relation to the substance may result in the substance becoming toxic within the meaning of the Act,
Therefore, the Minister of the Environment indicates, pursuant to section 85 of the Canadian Environmental Protection Act, 1999, that subsection 81(4) of that Act applies with respect to the substance in accordance with the Annex.
The Honourable Catherine McKenna
Minister of the Environment
ANNEX
Information requirements
(Section 85 of the Canadian Environmental Protection Act, 1999)
- The following definition applies in this notice:
“substance” means quaternary ammonium compounds, benzylalkyldimethyl, salts with bentonite, Confidential Accession No. 19214-2. - In relation to the substance, a significant new activity is any of the following:
- (a) any use of the substance in a quantity that exceeds 100 kg, but is equal to or less than 1 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres);
- (b) any use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year to manufacture any of the following products where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres):
- (i) a consumer product to which the Canada Consumer Product Safety Act applies,
- (ii) a natural health product as defined in subsection 1(1) of the Natural Health Products Regulations,
- (iii) a drug as defined in section 2 of the Food and Drugs Act, or
- (iv) a cosmetic as defined in section 2 of the Food and Drugs Act;
- (c) any other use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres); and
- (d) any use of the substance in a quantity that exceeds 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres).
- Section 2 does not apply to the use of the substance as a research and development substance or as a site-limited intermediate substance as these terms are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers), or as an export-only substance.
- The following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) for a significant new activity described in paragraph 2(a),
- (i) a description of the proposed significant new activity in relation to the substance,
- (ii) the anticipated annual quantity of the substance to be used in relation to the significant new activity,
- (iii) the information specified in items 2 and 7 of Schedule 4 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iv) the analytical information that is necessary to determine the primary and secondary particle size distribution of the substance (i.e. length, width and thickness), and
- (v) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance;
- (b) for a significant new activity described in paragraph 2(b),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with the Organisation for Economic Co-operation and Development (OECD) Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iii) the test data and the test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (iv) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study,
- (v) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study,
- (vi) if the significant new activity involves dermal exposure to the substance, the test data and a test report from a 28-day repeated dose dermal mammalian toxicity study that is conducted in accordance with OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, which is current at the time of the study, and
- (vii) if the significant new activity involves a spray application of the substance,
- (A) the test data and a test report from an in vivo 90-day repeated dose mammalian inhalation study that is conducted in accordance with OECD Test No. 413: Subchronic Inhalation Toxicity: 90-Day Study, which is current at the time of the study, including a satellite (reversibility) study, and with histopathological evaluation performed for all tissues and organs, and
- (B) the test data and test report from a study on bronchoalveolar lavage conducted following the last exposure during the subchronic inhalation toxicity test required under clause (A) that is conducted in accordance with the OECD Series on Testing and Assessment, No. 125: Guidance Document on Histopathology for Inhalation Toxicity Studies, Supporting TG 412 (Subacute Inhalation Toxicity: 28-Day Study) and TG 413 (Subchronic Inhalation Toxicity: 90-Day Study), which is current at the time of the study;
- (c) for a significant new activity described in paragraph 2(c),
- (i) the information mentioned in paragraph (a),
- (ii) the information specified in items 3 and 8 of Schedule 5 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from an acute inhalation toxicity study that is conducted in accordance with the OECD Test No. 403: Acute Inhalation Toxicity, which is current at the time of the study, and
- (iv) the test data and test report from an in vitro mammalian cell gene mutation study with and without metabolic activation that is conducted in accordance with OECD Test No. 476: In vitro Mammalian Cell Gene Mutation Tests Using the Hprt and xprt Genes, or OECD Test No. 490: In vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, which are current at the time of the study; and
- (d) for a significant new activity described in paragraph 2(d),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the information specified in items 2 to 4 of Schedule 6 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with OECD Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iv) the test data and test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency that is conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (v) the test data and the test report from a 28-day repeated dose mammalian toxicity study for the most significant route of potential human exposure to the substance that is conducted in accordance with OECD Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, or OECD Test 412: Subacute Inhalation Toxicity: 28-Day Study, which are current at the time of the study,
- (vi) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study, and
- (vii) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study.
- (a) for a significant new activity described in paragraph 2(a),
- The test data and the test reports referred to in section 4 must be developed in accordance with the OECD Principles of Good Laboratory Practice set out in Annex II of the Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted on May 12, 1981, by the OECD, using the Principles of Good Laboratory Practice that are current at the time the study is conducted. Furthermore, all studies regarding the substance must be conducted in accordance with the principles described in the following guidance documents, as amended from time to time:
- (a) Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials (OECD Series on the Safety of Manufactured Nanomaterials, No. 36);
- (b) Report of the OECD Expert Meeting on the Physical Chemical Properties of Manufactured Nanomaterials and Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 41); and
- (c) Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 40).
- For each study referred to in section 4, the following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) the analytical information that is necessary to determine the primary and secondary particle size of the substance used in the study (i.e. length, width and thickness); and
- (b) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance used in the study.
- The above-mentioned information will be assessed within 90 days after the day on which it is received by the Minister of the Environment.
EXPLANATORY NOTE
(This explanatory note is not part of the Significant New Activity Notice.)
Description
This Significant New Activity Notice is a legal instrument adopted by the Minister of the Environment pursuant to section 85 of the Canadian Environmental Protection Act, 1999 (CEPA) to apply the Significant New Activity (SNAc) provisions of that Act to quaternary ammonium compounds, benzylalkyldimethyl, salts with bentonite, Confidential Accession No. 19214-2. The Notice is now in force. It is therefore mandatory to meet all the requirements of the Notice should a person intend to use the substance for a significant new activity as defined in the Notice. (see footnote 5)
A Significant New Activity Notice does not constitute an endorsement from the Department of the Environment or the Government of Canada of the substance to which it relates, or an exemption from any other laws or regulations that are in force in Canada and that may apply to this substance or activities involving the substance.
Applicability of the Significant New Activity Notice
The Notice requires that any person (individual or corporation) engaging in a significant new activity in relation to quaternary ammonium compounds, benzylalkyldimethyl, salts with bentonite, Confidential Accession No. 19214-2, submit a Significant New Activity notification (SNAN) containing all of the information prescribed in the Notice at least 90 days prior to using the substance for the significant new activity.
In order to address human health concerns, the Notice targets the use of the substance containing particles at the nanoscale (1–100 nanometres) in commercial manufacturing activities. The activities include the use of the substance to manufacture consumer products to which the Canada Consumer Product Safety Act (CCPSA) (see footnote 6) applies, natural health products as defined in subsection 1(1) of the Natural Health Products Regulations (see footnote 7) or any drug or cosmetic as defined in the Food and Drugs Act. (see footnote 8)
For any other activity related to any of these uses, notification is required when, during a calendar year, the total quantity of the substance containing particles at the nanoscale (1–100 nanometres) in the product is greater than the specified trigger quantities mentioned in the Notice. For example, notification is required if a person plans to use the substance containing particles at the nanoscale (1–100 nanometres) where there is more than 100 kg of the substance involved in a calendar year.
Activities not subject to the Notice
Activities involving the use of the substance as a research and development substance, a site-limited intermediate or an export-only substance are excluded from the Notice. The terms “research and development substance” and “site-limited intermediate substance” are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 9) An export-only substance is a substance that is manufactured in or imported into Canada and destined solely for foreign markets.
This Notice does not apply to uses of the substance that are regulated under the acts of Parliament listed in Schedule 2 of CEPA, including the Pest Control Products Act, the Fertilizers Act and the Feeds Act. The Notice also does not apply to transient reaction intermediates, impurities, contaminants, partially unreacted materials, or in some circumstances to items such as, but not limited to, wastes, mixtures, or manufactured items. However, it should be noted that individual components of a mixture may be subject to notification under the SNAc provisions of CEPA. See subsection 81(6) and section 3 of CEPA, and section 3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers for additional information. (see footnote 10)
Information to be submitted
The Notice sets out the information that must be provided to the Minister 90 days before the day on which the substance quaternary ammonium compounds, benzylalkyldimethyl, salts with bentonite, Confidential Accession No. 19214-2, is used for a significant new activity. The Department of the Environment and the Department of Health will use the information submitted in the SNAN to conduct human health and environmental assessments within 90 days after the complete information is received.
The assessment of the substance identified concerns associated with potential activities involving use of the substance containing particles at the nanoscale thereby resulting in exposure and toxicity to human health. The Significant New Activity Notice is issued to obtain information to ensure that the substance will undergo further assessment before significant new activities are undertaken.
The information requirements in the Notice relate to general information in respect of the substance, details surrounding its use, exposure information, and toxicity to human health. Some of the information requirements reference the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 11)
Additional guidance on preparing a SNAN can be found in section 1.3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers. (see footnote 12)
Compliance
When assessing whether or not a substance is subject to SNAc provisions, (see footnote 13) a person is expected to make use of information in their possession or to which they ought to have access. The phrase “to which they ought to have access” means information in any of the notifier's offices worldwide or other locations where the notifier can reasonably have access to the information. For example, manufacturers are expected to have access to their formulations, while importers or users of a substance, mixture, or product are expected to have access to import records, usage information and the relevant Safety Data Sheets (SDSs). (see footnote 14)
Although an SDS is an important source of information on the composition of a product, it should be noted that the goal of the SDS is to protect the health of workers in the workplace from specific hazards of chemical products. Therefore, an SDS may not list all product ingredients that may be subject to a SNAc notice due to toxicity to human health or the environment. Any person requiring more detailed information on product composition is encouraged to contact their supplier.
If any information becomes available that reasonably supports the conclusion that the substance quaternary ammonium compounds, benzylalkyldimethyl, salts with bentonite, Confidential Accession No. 19214-2, is toxic or capable of becoming toxic, the person who is in possession of the information and is involved in activities with the substance is obligated, under section 70 of CEPA, to provide that information to the Minister without delay.
A company can submit a SNAN on behalf of its clients. For example, in cases where a person takes possession or control of a substance from another person, they may not be required to submit a SNAN, under certain conditions, if their activities were covered by an original SNAN submitted by the person from whom they obtained the substance. The Substances Management Advisory Note “Clarification in relation to the submission of Significant New Activity Notifications in application of the Canadian Environmental Protection Act, 1999” provides more detail on this subject. (see footnote 15)
Under section 86 of CEPA, any person who transfers the physical possession or control of a substance subject to a SNAc notice shall notify all persons to whom the physical possession or control is transferred of the obligation to comply with the notice, including the obligation to notify the Minister of any significant new activity and to provide all the required information outlined above.
A pre-notification consultation (PNC) is recommended for notifiers who wish to consult with the program during the planning or preparation of their SNAN to discuss any questions or concerns they have about the prescribed information and test plans.
Where a person has questions concerning their obligations to comply with a notice, believes they may be out of compliance, or would like to request a PNC, they are encouraged to discuss their particular circumstances with the program by contacting the Substances Management Information Line. (see footnote 16)
CEPA is enforced in accordance with the publicly available Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999. (see footnote 17) In instances of non-compliance, consideration is given to factors such as the nature of the alleged violation, potential harm, intent, and history of compliance.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Significant New Activity Notice No. 19180
Significant New Activity Notice
(Section 85 of the Canadian Environmental Protection Act, 1999)
Whereas the Minister of the Environment and the Minister of Health have assessed information in respect of the substance quaternary ammonium compounds, dialkyldimethyl, salts with bentonite, Confidential Accession No. 19215-3, under section 83 of the Canadian Environmental Protection Act, 1999;
Whereas the substance is not specified on the Domestic Substances List;
And whereas the ministers suspect that a significant new activity in relation to the substance may result in the substance becoming toxic within the meaning of the Act,
Therefore, the Minister of the Environment indicates, pursuant to section 85 of the Canadian Environmental Protection Act, 1999, that subsection 81(4) of that Act applies with respect to the substance in accordance with the Annex.
The Honourable Catherine McKenna
Minister of the Environment
ANNEX
Information requirements
(Section 85 of the Canadian Environmental Protection Act, 1999)
- The following definition applies in this notice:
“substance” means quaternary ammonium compounds, dialkyldimethyl, salts with bentonite, Confidential Accession No. 19215-3. - In relation to the substance, a significant new activity is any of the following:
- (a) any use of the substance in a quantity that exceeds 100 kg, but is equal to or less than 1 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres);
- (b) any use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year to manufacture any of the following products where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres):
- (i) a consumer product to which the Canada Consumer Product Safety Act applies,
- (ii) a natural health product as defined in subsection 1(1) of the Natural Health Products Regulations,
- (iii) a drug as defined in section 2 of the Food and Drugs Act, or
- (iv) a cosmetic as defined in section 2 of the Food and Drugs Act;
- (c) any other use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres); and
- (d) any use of the substance in a quantity that exceeds 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres).
- Section 2 does not apply to the use of the substance as a research and development substance or as a site-limited intermediate substance as these terms are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers), or as an export-only substance.
- The following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) for a significant new activity described in paragraph 2(a),
- (i) a description of the proposed significant new activity in relation to the substance,
- (ii) the anticipated annual quantity of the substance to be used in relation to the significant new activity,
- (iii) the information specified in items 2 and 7 of Schedule 4 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iv) the analytical information that is necessary to determine the primary and secondary particle size distribution of the substance (i.e. length, width and thickness), and
- (v) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance;
- (b) for a significant new activity described in paragraph 2(b),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with the Organisation for Economic Co-operation and Development (OECD) Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iii) the test data and the test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (iv) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study,
- (v) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study,
- (vi) if the significant new activity involves dermal exposure to the substance, the test data and a test report from a 28-day repeated dose dermal mammalian toxicity study that is conducted in accordance with OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, which is current at the time of the study, and
- (vii) if the significant new activity involves a spray application of the substance,
- (A) the test data and a test report from an in vivo 90-day repeated dose mammalian inhalation study that is conducted in accordance with OECD Test No. 413: Subchronic Inhalation Toxicity: 90-Day Study, which is current at the time of the study, including a satellite (reversibility) study, and with histopathological evaluation performed for all tissues and organs, and
- (B) the test data and test report from a study on bronchoalveolar lavage conducted following the last exposure during the subchronic inhalation toxicity test required under clause (A) that is conducted in accordance with the OECD Series on Testing and Assessment, No. 125: Guidance Document on Histopathology for Inhalation Toxicity Studies, Supporting TG 412 (Subacute Inhalation Toxicity: 28-Day Study) and TG 413 (Subchronic Inhalation Toxicity: 90-Day Study), which is current at the time of the study;
- (c) for a significant new activity described in paragraph 2(c),
- (i) the information mentioned in paragraph (a),
- (ii) the information specified in items 3 and 8 of Schedule 5 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from an acute inhalation toxicity study that is conducted in accordance with the OECD Test No. 403: Acute Inhalation Toxicity, which is current at the time of the study, and
- (iv) the test data and test report from an in vitro mammalian cell gene mutation study with and without metabolic activation that is conducted in accordance with OECD Test No. 476: In vitro Mammalian Cell Gene Mutation Tests Using the Hprt and xprt Genes, or OECD Test No. 490: In vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, which are current at the time of the study; and
- (d) for a significant new activity described in paragraph 2(d),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the information specified in items 2 to 4 of Schedule 6 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with OECD Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iv) the test data and test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency that is conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (v) the test data and the test report from a 28-day repeated dose mammalian toxicity study for the most significant route of potential human exposure to the substance that is conducted in accordance with OECD Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, or OECD Test 412: Subacute Inhalation Toxicity: 28-Day Study, which are current at the time of the study,
- (vi) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study, and
- (vii) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study.
- (a) for a significant new activity described in paragraph 2(a),
- The test data and the test reports referred to in section 4 must be developed in accordance with the OECD Principles of Good Laboratory Practice set out in Annex II of the Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted on May 12, 1981, by the OECD, using the Principles of Good Laboratory Practice that are current at the time the study is conducted. Furthermore, all studies regarding the substance must be conducted in accordance with the principles described in the following guidance documents, as amended from time to time:
- (a) Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials (OECD Series on the Safety of Manufactured Nanomaterials, No. 36);
- (b) Report of the OECD Expert Meeting on the Physical Chemical Properties of Manufactured Nanomaterials and Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 41); and
- (c) Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 40).
- For each study referred to in section 4, the following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) the analytical information that is necessary to determine the primary and secondary particle size of the substance used in the study (i.e. length, width and thickness); and
- (b) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance used in the study.
- The above-mentioned information will be assessed within 90 days after the day on which it is received by the Minister of the Environment.
EXPLANATORY NOTE
(This explanatory note is not part of the Significant New Activity Notice.)
Description
This Significant New Activity Notice is a legal instrument adopted by the Minister of the Environment pursuant to section 85 of the Canadian Environmental Protection Act, 1999 (CEPA) to apply the Significant New Activity (SNAc) provisions of that Act to quaternary ammonium compounds, dialkyldimethyl, salts with bentonite, Confidential Accession No. 19215-3. The Notice is now in force. It is therefore mandatory to meet all the requirements of the Notice should a person intend to use the substance for a significant new activity as defined in the Notice. (see footnote 18)
A Significant New Activity Notice does not constitute an endorsement from the Department of the Environment or the Government of Canada of the substance to which it relates, or an exemption from any other laws or regulations that are in force in Canada and that may apply to this substance or activities involving the substance.
Applicability of the Significant New Activity Notice
The Notice requires that any person (individual or corporation) engaging in a significant new activity in relation to quaternary ammonium compounds, dialkyldimethyl, salts with bentonite, Confidential Accession No. 19215-3, submit a Significant New Activity notification (SNAN) containing all of the information prescribed in the Notice at least 90 days prior to using the substance for the significant new activity.
In order to address human health concerns, the Notice targets the use of the substance containing particles at the nanoscale (1–100 nanometres) in commercial manufacturing activities. The activities include the use of the substance to manufacture consumer products to which the Canada Consumer Product Safety Act (CCPSA) (see footnote 19) applies, natural health products as defined in subsection 1(1) of the Natural Health Products Regulations (see footnote 20) or any drug or cosmetic as defined in the Food and Drugs Act. (see footnote 21)
For any other activity related to any of these uses, notification is required when, during a calendar year, the total quantity of the substance containing particles at the nanoscale (1–100 nanometres) in the product is greater than the specified trigger quantities mentioned in the Notice. For example, notification is required if a person plans to use the substance containing particles at the nanoscale (1–100 nanometres) where there is more than 100 kg of the substance involved in a calendar year.
Activities not subject to the Notice
Activities involving the use of the substance as a research and development substance, a site-limited intermediate or an export-only substance are excluded from the Notice. The terms “research and development substance” and “site-limited intermediate substance” are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 22) An export-only substance is a substance that is manufactured in or imported into Canada and destined solely for foreign markets.
This Notice does not apply to uses of the substance that are regulated under the acts of Parliament listed in Schedule 2 of CEPA, including the Pest Control Products Act, the Fertilizers Act and the Feeds Act. The Notice also does not apply to transient reaction intermediates, impurities, contaminants, partially unreacted materials, or in some circumstances to items such as, but not limited to, wastes, mixtures, or manufactured items. However, it should be noted that individual components of a mixture may be subject to notification under the SNAc provisions of CEPA. See subsection 81(6) and section 3 of CEPA, and section 3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers for additional information. (see footnote 23)
Information to be submitted
The Notice sets out the information that must be provided to the Minister 90 days before the day on which the quaternary ammonium compounds, dialkyldimethyl, salts with bentonite, Confidential Accession No. 19215-3, is used for a significant new activity. The Department of the Environment and the Department of Health will use the information submitted in the SNAN to conduct human health and environmental assessments within 90 days after the complete information is received.
The assessment of the substance identified concerns associated with potential activities involving use of the substance containing particles at the nanoscale thereby resulting in exposure and toxicity to human health. The Significant New Activity Notice is issued to obtain information to ensure that the substance will undergo further assessment before significant new activities are undertaken.
The information requirements in the Notice relate to general information in respect of the substance, details surrounding its use, exposure information, and toxicity to human health. Some of the information requirements reference the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 24)
Additional guidance on preparing a SNAN can be found in section 1.3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers. (see footnote 25)
Compliance
When assessing whether or not a substance is subject to SNAc provisions, (see footnote 26) a person is expected to make use of information in their possession or to which they ought to have access. The phrase “to which they ought to have access” means information in any of the notifier's offices worldwide or other locations where the notifier can reasonably have access to the information. For example, manufacturers are expected to have access to their formulations, while importers or users of a substance, mixture, or product are expected to have access to import records, usage information and the relevant Safety Data Sheets (SDSs). (see footnote 27)
Although an SDS is an important source of information on the composition of a product, it should be noted that the goal of the SDS is to protect the health of workers in the workplace from specific hazards of chemical products. Therefore, an SDS may not list all product ingredients that may be subject to a SNAc notice due to toxicity to human health or the environment. Any person requiring more detailed information on product composition is encouraged to contact their supplier.
If any information becomes available that reasonably supports the conclusion that the substance quaternary ammonium compounds, dialkyldimethyl, salts with bentonite, Confidential Accession No. 19215-3, is toxic or capable of becoming toxic, the person who is in possession of the information and is involved in activities with the substance is obligated, under section 70 of CEPA, to provide that information to the Minister without delay.
A company can submit a SNAN on behalf of its clients. For example, in cases where a person takes possession or control of a substance from another person, they may not be required to submit a SNAN, under certain conditions, if their activities were covered by an original SNAN submitted by the person from whom they obtained the substance. The Substances Management Advisory Note “Clarification in relation to the submission of Significant New Activity Notifications in application of the Canadian Environmental Protection Act, 1999” provides more detail on this subject. (see footnote 28)
Under section 86 of CEPA, any person who transfers the physical possession or control of a substance subject to a SNAc notice shall notify all persons to whom the physical possession or control is transferred of the obligation to comply with the notice, including the obligation to notify the Minister of any significant new activity and to provide all the required information outlined above.
A pre-notification consultation (PNC) is recommended for notifiers who wish to consult with the program during the planning or preparation of their SNAN to discuss any questions or concerns they have about the prescribed information and test plans.
Where a person has questions concerning their obligations to comply with a notice, believes they may be out of compliance, or would like to request a PNC, they are encouraged to discuss their particular circumstances with the program by contacting the Substances Management Information Line. (see footnote 29)
CEPA is enforced in accordance with the publicly available Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999. (see footnote 30) In instances of non-compliance, consideration is given to factors such as the nature of the alleged violation, potential harm, intent, and history of compliance.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Significant New Activity Notice No. 19182
Significant New Activity Notice
(Section 85 of the Canadian Environmental Protection Act, 1999)
Whereas the Minister of the Environment and the Minister of Health have assessed information in respect of the substance quaternary ammonium compounds, bis(derivative oil alkyl)dimethyl, salts with smectite group minerals, Confidential Accession No. 19216-4, under section 83 of the Canadian Environmental Protection Act, 1999;
Whereas the substance is not specified on the Domestic Substances List;
And whereas the ministers suspect that a significant new activity in relation to the substance may result in the substance becoming toxic within the meaning of the Act,
Therefore, the Minister of the Environment indicates, pursuant to section 85 of the Canadian Environmental Protection Act, 1999, that subsection 81(4) of that Act applies with respect to the substance in accordance with the Annex.
The Honourable Catherine McKenna
Minister of the Environment
ANNEX
Information requirements
(Section 85 of the Canadian Environmental Protection Act, 1999)
- The following definition applies in this notice:
“substance” means quaternary ammonium compounds, bis(derivative oil alkyl)dimethyl, salts with smectite group minerals, Confidential Accession No. 19216-4. - In relation to the substance, a significant new activity is any of the following:
- (a) any use of the substance in a quantity that exceeds 100 kg, but is equal to or less than 1 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres);
- (b) any use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year to manufacture any of the following products where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres):
- (i) a consumer product to which the Canada Consumer Product Safety Act applies,
- (ii) a natural health product as defined in subsection 1(1) of the Natural Health Products Regulations,
- (iii) a drug as defined in section 2 of the Food and Drugs Act, or
- (iv) a cosmetic as defined in section 2 of the Food and Drugs Act;
- (c) any other use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres); and
- (d) any use of the substance in a quantity that exceeds 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres).
- Section 2 does not apply to the use of the substance as a research and development substance or as a site-limited intermediate substance as these terms are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers), or as an export-only substance.
- The following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) for a significant new activity described in paragraph 2(a),
- (i) a description of the proposed significant new activity in relation to the substance,
- (ii) the anticipated annual quantity of the substance to be used in relation to the significant new activity,
- (iii) the information specified in items 2 and 7 of Schedule 4 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iv) the analytical information that is necessary to determine the primary and secondary particle size distribution of the substance (i.e. length, width and thickness), and
- (v) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance;
- (b) for a significant new activity described in paragraph 2(b),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with the Organisation for Economic Co-operation and Development (OECD) Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iii) the test data and the test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (iv) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study,
- (v) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study,
- (vi) if the significant new activity involves dermal exposure to the substance, the test data and a test report from a 28-day repeated dose dermal mammalian toxicity study that is conducted in accordance with OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, which is current at the time of the study, and
- (vii) if the significant new activity involves a spray application of the substance,
- (A) the test data and a test report from an in vivo 90-day repeated dose mammalian inhalation study that is conducted in accordance with OECD Test No. 413: Subchronic Inhalation Toxicity: 90-Day Study, which is current at the time of the study, including a satellite (reversibility) study, and with histopathological evaluation performed for all tissues and organs, and
- (B) the test data and test report from a study on bronchoalveolar lavage conducted following the last exposure during the subchronic inhalation toxicity test required under clause (A) that is conducted in accordance with the OECD Series on Testing and Assessment, No. 125: Guidance Document on Histopathology for Inhalation Toxicity Studies, Supporting TG 412 (Subacute Inhalation Toxicity: 28-Day Study) and TG 413 (Subchronic Inhalation Toxicity: 90-Day Study), which is current at the time of the study;
- (c) for a significant new activity described in paragraph 2(c),
- (i) the information mentioned in paragraph (a),
- (ii) the information specified in items 3 and 8 of Schedule 5 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from an acute inhalation toxicity study that is conducted in accordance with the OECD Test No. 403: Acute Inhalation Toxicity, which is current at the time of the study, and
- (iv) the test data and test report from an in vitro mammalian cell gene mutation study with and without metabolic activation that is conducted in accordance with OECD Test No. 476: In vitro Mammalian Cell Gene Mutation Tests Using the Hprt and xprt Genes, or OECD Test No. 490: In vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, which are current at the time of the study; and
- (d) for a significant new activity described in paragraph 2(d),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the information specified in items 2 to 4 of Schedule 6 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with OECD Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iv) the test data and test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency that is conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (v) the test data and the test report from a 28-day repeated dose mammalian toxicity study for the most significant route of potential human exposure to the substance that is conducted in accordance with OECD Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, or OECD Test 412: Subacute Inhalation Toxicity: 28-Day Study, which are current at the time of the study,
- (vi) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study, and
- (vii) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study.
- (a) for a significant new activity described in paragraph 2(a),
- The test data and the test reports referred to in section 4 must be developed in accordance with the OECD Principles of Good Laboratory Practice set out in Annex II of the Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted on May 12, 1981, by the OECD, using the Principles of Good Laboratory Practice that are current at the time the study is conducted. Furthermore, all studies regarding the substance must be conducted in accordance with the principles described in the following guidance documents, as amended from time to time:
- (a) Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials (OECD Series on the Safety of Manufactured Nanomaterials, No. 36);
- (b) Report of the OECD Expert Meeting on the Physical Chemical Properties of Manufactured Nanomaterials and Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 41); and
- (c) Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 40).
- For each study referred to in section 4, the following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) the analytical information that is necessary to determine the primary and secondary particle size of the substance used in the study (i.e. length, width and thickness); and
- (b) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance used in the study.
- The above-mentioned information will be assessed within 90 days after the day on which it is received by the Minister of the Environment.
EXPLANATORY NOTE
(This explanatory note is not part of the Significant New Activity Notice.)
Description
This Significant New Activity Notice is a legal instrument adopted by the Minister of the Environment pursuant to section 85 of the Canadian Environmental Protection Act, 1999 (CEPA) to apply the Significant New Activity (SNAc) provisions of that Act to quaternary ammonium compounds, bis(derivative oil alkyl)dimethyl, salts with smectite group minerals, Confidential Accession No. 19216-4. The Notice is now in force. It is therefore mandatory to meet all the requirements of the Notice should a person intend to use the substance for a significant new activity as defined in the Notice. (see footnote 31)
A Significant New Activity Notice does not constitute an endorsement from the Department of the Environment or the Government of Canada of the substance to which it relates, or an exemption from any other laws or regulations that are in force in Canada and that may apply to this substance or activities involving the substance.
Applicability of the Significant New Activity Notice
The Notice requires that any person (individual or corporation) engaging in a significant new activity in relation to quaternary ammonium compounds, bis(derivative oil alkyl)dimethyl, salts with smectite group minerals, Confidential Accession No. 19216-4, submit a Significant New Activity notification (SNAN) containing all of the information prescribed in the Notice at least 90 days prior to using the substance for the significant new activity.
In order to address human health concerns, the Notice targets the use of the substance containing particles at the nanoscale (1–100 nanometres) in commercial manufacturing activities. The activities include the use of the substance to manufacture consumer products to which the Canada Consumer Product Safety Act (CCPSA) (see footnote 32) applies, natural health products as defined in subsection 1(1) of the Natural Health Products Regulations (see footnote 33) or any drug or cosmetic as defined in the Food and Drugs Act. (see footnote 34)
For any other activity related to any of these uses, notification is required when, during a calendar year, the total quantity of the substance containing particles at the nanoscale (1–100 nanometres) in the product is greater than the specified trigger quantities mentioned in the Notice. For example, notification is required if a person plans to use the substance containing particles at the nanoscale (1–100 nanometres) where there is more than 100 kg of the substance involved in a calendar year.
Activities not subject to the Notice
Activities involving the use of the substance as a research and development substance, a site-limited intermediate or an export-only substance are excluded from the Notice. The terms “research and development substance” and “site-limited intermediate substance” are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 35) An export-only substance is a substance that is manufactured in or imported into Canada and destined solely for foreign markets.
This Notice does not apply to uses of the substance that are regulated under the acts of Parliament listed in Schedule 2 of CEPA, including the Pest Control Products Act, the Fertilizers Act and the Feeds Act. The Notice also does not apply to transient reaction intermediates, impurities, contaminants, partially unreacted materials, or in some circumstances to items such as, but not limited to, wastes, mixtures, or manufactured items. However, it should be noted that individual components of a mixture may be subject to notification under the SNAc provisions of CEPA. See subsection 81(6) and section 3 of CEPA, and section 3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers for additional information. (see footnote 36)
Information to be submitted
The Notice sets out the information that must be provided to the Minister 90 days before the day on which the quaternary ammonium compounds, bis(derivative oil alkyl)dimethyl, salts with smectite group minerals, Confidential Accession No. 19216-4, is used for a significant new activity. The Department of the Environment and the Department of Health will use the information submitted in the SNAN to conduct human health and environmental assessments within 90 days after the complete information is received.
The assessment of the substance identified concerns associated with potential activities involving use of the substance containing particles at the nanoscale thereby resulting in exposure and toxicity to human health. The Significant New Activity Notice is issued to obtain information to ensure that the substance will undergo further assessment before significant new activities are undertaken.
The information requirements in the Notice relate to general information in respect of the substance, details surrounding its use, exposure information, and toxicity to human health. Some of the information requirements reference the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 37)
Additional guidance on preparing a SNAN can be found in section 1.3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers. (see footnote 38)
Compliance
When assessing whether or not a substance is subject to SNAc provisions, (see footnote 39) a person is expected to make use of information in their possession or to which they ought to have access. The phrase “to which they ought to have access” means information in any of the notifier's offices worldwide or other locations where the notifier can reasonably have access to the information. For example, manufacturers are expected to have access to their formulations, while importers or users of a substance, mixture, or product are expected to have access to import records, usage information and the relevant Safety Data Sheets (SDSs). (see footnote 40)
Although an SDS is an important source of information on the composition of a product, it should be noted that the goal of the SDS is to protect the health of workers in the workplace from specific hazards of chemical products. Therefore, an SDS may not list all product ingredients that may be subject to a SNAc notice due to toxicity to human health or the environment. Any person requiring more detailed information on product composition is encouraged to contact their supplier.
If any information becomes available that reasonably supports the conclusion that the substance quaternary ammonium compounds, bis(derivative oil alkyl)dimethyl, salts with smectite group minerals, Confidential Accession No. 19216-4, is toxic or capable of becoming toxic, the person who is in possession of the information and is involved in activities with the substance is obligated, under section 70 of CEPA, to provide that information to the Minister without delay.
A company can submit a SNAN on behalf of its clients. For example, in cases where a person takes possession or control of a substance from another person, they may not be required to submit a SNAN, under certain conditions, if their activities were covered by an original SNAN submitted by the person from whom they obtained the substance. The Substances Management Advisory Note “Clarification in relation to the submission of Significant New Activity Notifications in application of the Canadian Environmental Protection Act, 1999” provides more detail on this subject. (see footnote 41)
Under section 86 of CEPA, any person who transfers the physical possession or control of a substance subject to a SNAc notice shall notify all persons to whom the physical possession or control is transferred of the obligation to comply with the notice, including the obligation to notify the Minister of any significant new activity and to provide all the required information outlined above.
A pre-notification consultation (PNC) is recommended for notifiers who wish to consult with the program during the planning or preparation of their SNAN to discuss any questions or concerns they have about the prescribed information and test plans.
Where a person has questions concerning their obligations to comply with a notice, believes they may be out of compliance, or would like to request a PNC, they are encouraged to discuss their particular circumstances with the program by contacting the Substances Management Information Line. (see footnote 42)
CEPA is enforced in accordance with the publicly available Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999. (see footnote 43) In instances of non-compliance, consideration is given to factors such as the nature of the alleged violation, potential harm, intent, and history of compliance.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Significant New Activity Notice No. 19184
Significant New Activity Notice
(Section 85 of the Canadian Environmental Protection Act, 1999)
Whereas the Minister of the Environment and the Minister of Health have assessed information in respect of the substance quaternary ammonium compounds, benzylalkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19217-5, under section 83 of the Canadian Environmental Protection Act, 1999;
Whereas the substance is not specified on the Domestic Substances List;
And whereas the ministers suspect that a significant new activity in relation to the substance may result in the substance becoming toxic within the meaning of the Act,
Therefore, the Minister of the Environment indicates, pursuant to section 85 of the Canadian Environmental Protection Act, 1999, that subsection 81(4) of that Act applies with respect to the substance in accordance with the Annex.
The Honourable Catherine McKenna
Minister of the Environment
ANNEX
Information requirements
(Section 85 of the Canadian Environmental Protection Act, 1999)
- The following definition applies in this notice:
- “substance” means quaternary ammonium compounds, benzylalkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19217-5.
- In relation to the substance, a significant new activity is any of the following:
- (a) any use of the substance in a quantity that exceeds 100 kg, but is equal to or less than 1 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres);
- (b) any use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year to manufacture any of the following products where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres):
- (i) a consumer product to which the Canada Consumer Product Safety Act applies,
- (ii) a natural health product as defined in subsection 1(1) of the Natural Health Products Regulations,
- (iii) a drug as defined in section 2 of the Food and Drugs Act, or
- (iv) a cosmetic as defined in section 2 of the Food and Drugs Act;
- (c) any other use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres); and
- (d) any use of the substance in a quantity that exceeds 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres).
- Section 2 does not apply to the use of the substance as a research and development substance or as a site-limited intermediate substance as these terms are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers), or as an export-only substance.
- The following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) for a significant new activity described in paragraph 2(a),
- (i) a description of the proposed significant new activity in relation to the substance,
- (ii) the anticipated annual quantity of the substance to be used in relation to the significant new activity,
- (iii) the information specified in items 2 and 7 of Schedule 4 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iv) the analytical information that is necessary to determine the primary and secondary particle size distribution of the substance (i.e. length, width and thickness), and
- (v) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance;
- (b) for a significant new activity described in paragraph 2(b),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with the Organisation for Economic Co-operation and Development (OECD) Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iii) the test data and the test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (iv) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study,
- (v) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study,
- (vi) if the significant new activity involves dermal exposure to the substance, the test data and a test report from a 28-day repeated dose dermal mammalian toxicity study that is conducted in accordance with OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, which is current at the time of the study, and
- (vii) if the significant new activity involves a spray application of the substance,
- (A) the test data and a test report from an in vivo 90-day repeated dose mammalian inhalation study that is conducted in accordance with OECD Test No. 413: Subchronic Inhalation Toxicity: 90-Day Study, which is current at the time of the study, including a satellite (reversibility) study, and with histopathological evaluation performed for all tissues and organs, and
- (B) the test data and test report from a study on bronchoalveolar lavage conducted following the last exposure during the subchronic inhalation toxicity test required under clause (A) that is conducted in accordance with the OECD Series on Testing and Assessment, No. 125: Guidance Document on Histopathology for Inhalation Toxicity Studies, Supporting TG 412 (Subacute Inhalation Toxicity: 28-Day Study) and TG 413 (Subchronic Inhalation Toxicity: 90-Day Study), which is current at the time of the study;
- (c) for a significant new activity described in paragraph 2(c),
- (i) the information mentioned in paragraph (a),
- (ii) the information specified in items 3 and 8 of Schedule 5 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from an acute inhalation toxicity study that is conducted in accordance with the OECD Test No. 403: Acute Inhalation Toxicity, which is current at the time of the study, and
- (iv) the test data and test report from an in vitro mammalian cell gene mutation study with and without metabolic activation that is conducted in accordance with OECD Test No. 476: In vitro Mammalian Cell Gene Mutation Tests Using the Hprt and xprt Genes, or OECD Test No. 490: In vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, which are current at the time of the study; and
- (d) for a significant new activity described in paragraph 2(d),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the information specified in items 2 to 4 of Schedule 6 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with OECD Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iv) the test data and test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency that is conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (v) the test data and the test report from a 28-day repeated dose mammalian toxicity study for the most significant route of potential human exposure to the substance that is conducted in accordance with OECD Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, or OECD Test 412: Subacute Inhalation Toxicity: 28-Day Study, which are current at the time of the study,
- (vi) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study, and
- (vii) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study.
- (a) for a significant new activity described in paragraph 2(a),
- The test data and the test reports referred to in section 4 must be developed in accordance with the OECD Principles of Good Laboratory Practice set out in Annex II of the Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted on May 12, 1981, by the OECD, using the Principles of Good Laboratory Practice that are current at the time the study is conducted. Furthermore, all studies regarding the substance must be conducted in accordance with the principles described in the following guidance documents, as amended from time to time:
- (a) Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials (OECD Series on the Safety of Manufactured Nanomaterials, No. 36);
- (b) Report of the OECD Expert Meeting on the Physical Chemical Properties of Manufactured Nanomaterials and Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 41); and
- (c) Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 40).
- For each study referred to in section 4, the following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) the analytical information that is necessary to determine the primary and secondary particle size of the substance used in the study (i.e. length, width and thickness); and
- (b) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance used in the study.
- The above-mentioned information will be assessed within 90 days after the day on which it is received by the Minister of the Environment.
EXPLANATORY NOTE
(This explanatory note is not part of the Significant New Activity Notice.)
Description
This Significant New Activity Notice is a legal instrument adopted by the Minister of the Environment pursuant to section 85 of the Canadian Environmental Protection Act, 1999 (CEPA) to apply the Significant New Activity (SNAc) provisions of that Act to quaternary ammonium compounds, benzylalkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19217-5. The Notice is now in force. It is therefore mandatory to meet all the requirements of the Notice should a person intend to use the substance for a significant new activity as defined in the Notice. (see footnote 44)
A Significant New Activity Notice does not constitute an endorsement from the Department of the Environment or the Government of Canada of the substance to which it relates, or an exemption from any other laws or regulations that are in force in Canada and that may apply to this substance or activities involving the substance.
Applicability of the Significant New Activity Notice
The Notice requires that any person (individual or corporation) engaging in a significant new activity in relation to quaternary ammonium compounds, benzylalkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19217-5, submit a Significant New Activity notification (SNAN) containing all of the information prescribed in the Notice at least 90 days prior to using the substance for the significant new activity.
In order to address human health concerns, the Notice targets the use of the substance containing particles at the nanoscale (1–100 nanometres) in commercial manufacturing activities. The activities include the use of the substance to manufacture consumer products to which the Canada Consumer Product Safety Act (CCPSA) (see footnote 45) applies, natural health products as defined in subsection 1(1) of the Natural Health Products Regulations (see footnote 46) or any drug or cosmetic as defined in the Food and Drugs Act. (see footnote 47)
For any other activity related to any of these uses, notification is required when, during a calendar year, the total quantity of the substance containing particles at the nanoscale (1–100 nanometres) in the product is greater than the specified trigger quantities mentioned in the Notice. For example, notification is required if a person plans to use the substance containing particles at the nanoscale (1–100 nanometres) where there is more than 100 kg of the substance involved in a calendar year.
Activities not subject to the Notice
Activities involving the use of the substance as a research and development substance, a site-limited intermediate or an export-only substance are excluded from the Notice. The terms “research and development substance” and “site-limited intermediate substance” are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 48) An export-only substance is a substance that is manufactured in or imported into Canada and destined solely for foreign markets.
This Notice does not apply to uses of the substance that are regulated under the acts of Parliament listed in Schedule 2 of CEPA, including the Pest Control Products Act, the Fertilizers Act and the Feeds Act. The Notice also does not apply to transient reaction intermediates, impurities, contaminants, partially unreacted materials, or in some circumstances to items such as, but not limited to, wastes, mixtures, or manufactured items. However, it should be noted that individual components of a mixture may be subject to notification under the SNAc provisions of CEPA. See subsection 81(6) and section 3 of CEPA, and section 3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers for additional information. (see footnote 49)
Information to be submitted
The Notice sets out the information that must be provided to the Minister 90 days before the day on which the quaternary ammonium compounds, benzylalkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19217-5, is used for a significant new activity. The Department of the Environment and the Department of Health will use the information submitted in the SNAN to conduct human health and environmental assessments within 90 days after the complete information is received.
The assessment of the substance identified concerns associated with potential activities involving use of the substance containing particles at the nanoscale thereby resulting in exposure and toxicity to human health. The Significant New Activity Notice is issued to obtain information to ensure that the substance will undergo further assessment before significant new activities are undertaken.
The information requirements in the Notice relate to general information in respect of the substance, details surrounding its use, exposure information, and toxicity to human health. Some of the information requirements reference the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 50)
Additional guidance on preparing a SNAN can be found in section 1.3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers. (see footnote 51)
Compliance
When assessing whether or not a substance is subject to SNAc provisions, (see footnote 52) a person is expected to make use of information in their possession or to which they ought to have access. The phrase “to which they ought to have access” means information in any of the notifier's offices worldwide or other locations where the notifier can reasonably have access to the information. For example, manufacturers are expected to have access to their formulations, while importers or users of a substance, mixture, or product are expected to have access to import records, usage information and the relevant Safety Data Sheets (SDSs). (see footnote 53)
Although an SDS is an important source of information on the composition of a product, it should be noted that the goal of the SDS is to protect the health of workers in the workplace from specific hazards of chemical products. Therefore, an SDS may not list all product ingredients that may be subject to a SNAc notice due to toxicity to human health or the environment. Any person requiring more detailed information on product composition is encouraged to contact their supplier.
If any information becomes available that reasonably supports the conclusion that the substance quaternary ammonium compounds, benzylalkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19217-5, is toxic or capable of becoming toxic, the person who is in possession of the information and is involved in activities with the substance is obligated, under section 70 of CEPA, to provide that information to the Minister without delay.
A company can submit a SNAN on behalf of its clients. For example, in cases where a person takes possession or control of a substance from another person, they may not be required to submit a SNAN, under certain conditions, if their activities were covered by an original SNAN submitted by the person from whom they obtained the substance. The Substances Management Advisory Note “Clarification in relation to the submission of Significant New Activity Notifications in application of the Canadian Environmental Protection Act, 1999” provides more detail on this subject. (see footnote 54)
Under section 86 of CEPA, any person who transfers the physical possession or control of a substance subject to a SNAc notice shall notify all persons to whom the physical possession or control is transferred of the obligation to comply with the notice, including the obligation to notify the Minister of any significant new activity and to provide all the required information outlined above.
A pre-notification consultation (PNC) is recommended for notifiers who wish to consult with the program during the planning or preparation of their SNAN to discuss any questions or concerns they have about the prescribed information and test plans.
Where a person has questions concerning their obligations to comply with a notice, believes they may be out of compliance, or would like to request a PNC, they are encouraged to discuss their particular circumstances with the program by contacting the Substances Management Information Line. (see footnote 55)
CEPA is enforced in accordance with the publicly available Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999. (see footnote 56) In instances of non-compliance, consideration is given to factors such as the nature of the alleged violation, potential harm, intent, and history of compliance.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Significant New Activity Notice No. 19186
Significant New Activity Notice
(Section 85 of the Canadian Environmental Protection Act, 1999)
Whereas the Minister of the Environment and the Minister of Health have assessed information in respect of the substance quaternary ammonium compounds, dialkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19218-6, under section 83 of the Canadian Environmental Protection Act, 1999;
Whereas the substance is not specified on the Domestic Substances List;
And whereas the ministers suspect that a significant new activity in relation to the substance may result in the substance becoming toxic within the meaning of the Act,
Therefore, the Minister of the Environment indicates, pursuant to section 85 of the Canadian Environmental Protection Act, 1999, that subsection 81(4) of that Act applies with respect to the substance in accordance with the Annex.
The Honourable Catherine McKenna
Minister of the Environment
ANNEX
Information requirements
(Section 85 of the Canadian Environmental Protection Act, 1999)
- The following definition applies in this notice:
- “substance” means quaternary ammonium compounds, dialkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19218-6.
- In relation to the substance, a significant new activity is any of the following:
- (a) any use of the substance in a quantity that exceeds 100 kg, but is equal to or less than 1 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres);
- (b) any use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year to manufacture any of the following products where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres):
- (i) a consumer product to which the Canada Consumer Product Safety Act applies,
- (ii) a natural health product as defined in subsection 1(1) of the Natural Health Products Regulations,
- (iii) a drug as defined in section 2 of the Food and Drugs Act, or
- (iv) a cosmetic as defined in section 2 of the Food and Drugs Act;
- (c) any other use of the substance in a quantity that exceeds 1 000 kg, but is equal to or less than 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres); and
- (d) any use of the substance in a quantity that exceeds 10 000 kg in a calendar year where the substance contains particles at the nanoscale (i.e. 1 to 100 nanometres).
- Section 2 does not apply to the use of the substance as a research and development substance or as a site-limited intermediate substance as these terms are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers), or as an export-only substance.
- The following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) for a significant new activity described in paragraph 2(a),
- (i) a description of the proposed significant new activity in relation to the substance,
- (ii) the anticipated annual quantity of the substance to be used in relation to the significant new activity,
- (iii) the information specified in items 2 and 7 of Schedule 4 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iv) the analytical information that is necessary to determine the primary and secondary particle size distribution of the substance (i.e. length, width and thickness), and
- (v) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance;
- (b) for a significant new activity described in paragraph 2(b),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with the Organisation for Economic Co-operation and Development (OECD) Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iii) the test data and the test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (iv) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study,
- (v) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study,
- (vi) if the significant new activity involves dermal exposure to the substance, the test data and a test report from a 28-day repeated dose dermal mammalian toxicity study that is conducted in accordance with OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, which is current at the time of the study, and
- (vii) if the significant new activity involves a spray application of the substance,
- (A) the test data and a test report from an in vivo 90-day repeated dose mammalian inhalation study that is conducted in accordance with OECD Test No. 413: Subchronic Inhalation Toxicity: 90-Day Study, which is current at the time of the study, including a satellite (reversibility) study, and with histopathological evaluation performed for all tissues and organs, and
- (B) the test data and test report from a study on bronchoalveolar lavage conducted following the last exposure during the subchronic inhalation toxicity test required under clause (A) that is conducted in accordance with the OECD Series on Testing and Assessment, No. 125: Guidance Document on Histopathology for Inhalation Toxicity Studies, Supporting TG 412 (Subacute Inhalation Toxicity: 28-Day Study) and TG 413 (Subchronic Inhalation Toxicity: 90-Day Study), which is current at the time of the study;
- (c) for a significant new activity described in paragraph 2(c),
- (i) the information mentioned in paragraph (a),
- (ii) the information specified in items 3 and 8 of Schedule 5 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from an acute inhalation toxicity study that is conducted in accordance with the OECD Test No. 403: Acute Inhalation Toxicity, which is current at the time of the study, and
- (iv) the test data and test report from an in vitro mammalian cell gene mutation study with and without metabolic activation that is conducted in accordance with OECD Test No. 476: In vitro Mammalian Cell Gene Mutation Tests Using the Hprt and xprt Genes, or OECD Test No. 490: In vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, which are current at the time of the study; and
- (d) for a significant new activity described in paragraph 2(d),
- (i) the information mentioned in paragraphs (a) and (c),
- (ii) the information specified in items 2 to 4 of Schedule 6 to the New Substances Notification Regulations (Chemicals and Polymers),
- (iii) the test data and test report from a dermal acute toxicity study that is conducted in accordance with OECD Test No. 402: Acute Dermal Toxicity: Fixed Dose Procedure, which is current at the time of the study,
- (iv) the test data and test report from a skin sensitization study that establishes a dose-response relationship and allows an assessment of potency that is conducted in accordance with OECD Test No. 429: Skin Sensitization: Local Lymph Node Assay, which is current at the time of the study,
- (v) the test data and the test report from a 28-day repeated dose mammalian toxicity study for the most significant route of potential human exposure to the substance that is conducted in accordance with OECD Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Test No. 410: Repeated Dose Dermal Toxicity: 28-day Study, or OECD Test 412: Subacute Inhalation Toxicity: 28-Day Study, which are current at the time of the study,
- (vi) the test data and a test report from an in vitro genotoxicity study for chromosomal aberrations in mammalian cells that is conducted in accordance with OECD Test No. 473: In vitro Mammalian Chromosomal Aberration Test, which is current at the time of the study, and
- (vii) the test data and a test report from an in vivo mammalian genotoxicity study for chromosomal aberrations that is conducted in accordance with OECD Test No. 474: Mammalian Erythrocyte Micronucleus Test, which is current at the time of the study.
- (a) for a significant new activity described in paragraph 2(a),
- The test data and the test reports referred to in section 4 must be developed in accordance with the OECD Principles of Good Laboratory Practice set out in Annex II of the Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted on May 12, 1981, by the OECD, using the Principles of Good Laboratory Practice that are current at the time the study is conducted. Furthermore, all studies regarding the substance must be conducted in accordance with the principles described in the following guidance documents, as amended from time to time:
- (a) Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials (OECD Series on the Safety of Manufactured Nanomaterials, No. 36);
- (b) Report of the OECD Expert Meeting on the Physical Chemical Properties of Manufactured Nanomaterials and Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 41); and
- (c) Ecotoxicology and Environmental Fate of Manufactured Nanomaterials: Test Guidelines (OECD Series on the Safety of Manufactured Nanomaterials, No. 40).
- For each study referred to in section 4, the following information must be provided to the Minister of the Environment at least 90 days before the commencement of the proposed significant new activity:
- (a) the analytical information that is necessary to determine the primary and secondary particle size of the substance used in the study (i.e. length, width and thickness); and
- (b) the information that is necessary to determine the agglomeration and aggregation state, shape, surface area and surface charge of the substance used in the study.
- The above-mentioned information will be assessed within 90 days after the day on which it is received by the Minister of the Environment.
EXPLANATORY NOTE
(This explanatory note is not part of the Significant New Activity Notice.)
Description
This Significant New Activity Notice is a legal instrument adopted by the Minister of the Environment pursuant to section 85 of the Canadian Environmental Protection Act, 1999 (CEPA) to apply the Significant New Activity (SNAc) provisions of that Act to quaternary ammonium compounds, dialkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19218-6. The Notice is now in force. It is therefore mandatory to meet all the requirements of the Notice should a person intend to use the substance for a significant new activity as defined in the Notice. (see footnote 57)
A Significant New Activity Notice does not constitute an endorsement from the Department of the Environment or the Government of Canada of the substance to which it relates, or an exemption from any other laws or regulations that are in force in Canada and that may apply to this substance or activities involving the substance.
Applicability of the Significant New Activity Notice
The Notice requires that any person (individual or corporation) engaging in a significant new activity in relation to quaternary ammonium compounds, dialkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19218-6, submit a Significant New Activity notification (SNAN) containing all of the information prescribed in the Notice at least 90 days prior to using the substance for the significant new activity.
In order to address human health concerns, the Notice targets the use of the substance containing particles at the nanoscale (1–100 nanometres) in commercial manufacturing activities. The activities include the use of the substance to manufacture consumer products to which the Canada Consumer Product Safety Act (CCPSA) (see footnote 58) applies, natural health products as defined in subsection 1(1) of the Natural Health Products Regulations (see footnote 59) or any drug or cosmetic as defined in the Food and Drugs Act. (see footnote 60)
For any other activity related to any of these uses, notification is required when, during a calendar year, the total quantity of the substance containing particles at the nanoscale (1–100 nanometres) in the product is greater than the specified trigger quantities mentioned in the Notice. For example, notification is required if a person plans to use the substance containing particles at the nanoscale (1–100 nanometres) where there is more than 100 kg of the substance involved in a calendar year.
Activities not subject to the Notice
Activities involving the use of the substance as a research and development substance, a site-limited intermediate or an export-only substance are excluded from the Notice. The terms “research and development substance” and “site-limited intermediate substance” are defined in subsection 1(1) of the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 61) An export-only substance is a substance that is manufactured in or imported into Canada and destined solely for foreign markets.
This Notice does not apply to uses of the substance that are regulated under the acts of Parliament listed in Schedule 2 of CEPA, including the Pest Control Products Act, the Fertilizers Act and the Feeds Act. The Notice also does not apply to transient reaction intermediates, impurities, contaminants, partially unreacted materials, or in some circumstances to items such as, but not limited to, wastes, mixtures, or manufactured items. However, it should be noted that individual components of a mixture may be subject to notification under the SNAc provisions of CEPA. See subsection 81(6) and section 3 of CEPA, and section 3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers for additional information. (see footnote 62)
Information to be submitted
The Notice sets out the information that must be provided to the Minister 90 days before the day on which the substance quaternary ammonium compounds, dialkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19218-6, is used for a significant new activity. The Department of the Environment and the Department of Health will use the information submitted in the SNAN to conduct human health and environmental assessments within 90 days after the complete information is received.
The assessment of the substance identified concerns associated with potential activities involving use of the substance containing particles at the nanoscale thereby resulting in exposure and toxicity to human health. The Significant New Activity Notice is issued to obtain information to ensure that the substance will undergo further assessment before significant new activities are undertaken.
The information requirements in the Notice relate to general information in respect of the substance, details surrounding its use, exposure information, and toxicity to human health. Some of the information requirements reference the New Substances Notification Regulations (Chemicals and Polymers). (see footnote 63)
Additional guidance on preparing a SNAN can be found in section 1.3 of the Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers. (see footnote 64)
Compliance
When assessing whether or not a substance is subject to SNAc provisions, (see footnote 65) a person is expected to make use of information in their possession or to which they ought to have access. The phrase “to which they ought to have access” means information in any of the notifier's offices worldwide or other locations where the notifier can reasonably have access to the information. For example, manufacturers are expected to have access to their formulations, while importers or users of a substance, mixture, or product are expected to have access to import records, usage information and the relevant Safety Data Sheets (SDSs). (see footnote 66)
Although an SDS is an important source of information on the composition of a product, it should be noted that the goal of the SDS is to protect the health of workers in the workplace from specific hazards of chemical products. Therefore, an SDS may not list all product ingredients that may be subject to a SNAc notice due to toxicity to human health or the environment. Any person requiring more detailed information on product composition is encouraged to contact their supplier.
If any information becomes available that reasonably supports the conclusion that the substance quaternary ammonium compounds, dialkyldimethyl, salts with smectite group minerals, Confidential Accession No. 19218-6, is toxic or capable of becoming toxic, the person who is in possession of the information and is involved in activities with the substance is obligated, under section 70 of CEPA, to provide that information to the Minister without delay.
A company can submit a SNAN on behalf of its clients. For example, in cases where a person takes possession or control of a substance from another person, they may not be required to submit a SNAN, under certain conditions, if their activities were covered by an original SNAN submitted by the person from whom they obtained the substance. The Substances Management Advisory Note “Clarification in relation to the submission of Significant New Activity Notifications in application of the Canadian Environmental Protection Act, 1999” provides more detail on this subject. (see footnote 67)
Under section 86 of CEPA, any person who transfers the physical possession or control of a substance subject to a SNAc notice shall notify all persons to whom the physical possession or control is transferred of the obligation to comply with the notice, including the obligation to notify the Minister of any significant new activity and to provide all the required information outlined above.
A pre-notification consultation (PNC) is recommended for notifiers who wish to consult with the program during the planning or preparation of their SNAN to discuss any questions or concerns they have about the prescribed information and test plans.
Where a person has questions concerning their obligations to comply with a notice, believes they may be out of compliance, or would like to request a PNC, they are encouraged to discuss their particular circumstances with the program by contacting the Substances Management Information Line. (see footnote 68)
CEPA is enforced in accordance with the publicly available Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999. (see footnote 69) In instances of non-compliance, consideration is given to factors such as the nature of the alleged violation, potential harm, intent, and history of compliance.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Waiver of information requirements for substances (subsection 81(9) of the Canadian Environmental Protection Act, 1999)
Whereas any person who proposes to import or manufacture a substance that is not on the Domestic Substances List must provide to the Minister of the Environment the information required under subsection 81(1) of the Canadian Environmental Protection Act, 1999;
Whereas any person who proposes to use, manufacture or import for a significant new activity a substance that is on the Domestic Substances List must provide to the Minister of the Environment the information required under subsection 81(3) of the Canadian Environmental Protection Act, 1999;
Whereas any person who proposes to use for a significant new activity a substance that is not on the Domestic Substances List must provide to the Minister of the Environment the information required under subsection 81(4) of the Canadian Environmental Protection Act, 1999;
Whereas a person may, pursuant to subsection 81(8) of the Canadian Environmental Protection Act, 1999, request any of the requirements to provide information under subsection 81(1), (3) or (4) of the Canadian Environmental Protection Act, 1999 to be waived;
Whereas a waiver may be granted by the Minister of the Environment under subsection 81(8) of the Canadian Environmental Protection Act, 1999 if
- (a) in the opinion of the Ministers, the information is not needed in order to determine whether the substance is toxic or capable of becoming toxic;
- (b) the substance is to be used for a prescribed purpose or manufactured at a location where, in the opinion of the Ministers, the person requesting the waiver is able to contain the substance so as to satisfactorily protect the environment and human health; or
- (c) it is not, in the opinion of the Ministers, practicable or feasible to obtain the test data necessary to generate the information;
Therefore, notice is hereby given pursuant to subsection 81(9) of the Canadian Environmental Protection Act, 1999 that the Minister of the Environment waived some requirements to provide information in accordance with the following annex pursuant to subsection 81(8) of that Act.
Julie Thompson
Executive Director
Program Development and Engagement Division
On behalf of the Minister of the Environment
ANNEX
Waiver of information requirements
| Person to whom a waiver was granted | Information concerning a substance in relation to which a waiver was granted |
|---|---|
Cegene Inc. |
Data in respect of vapour pressure |
Chemroy Canada Inc. |
Data in respect of octanol–water partition coefficient Data in respect of hydrolysis rate as a function of pH (3) (see note 1) Data from an in vivo mammalian mutagenicity test (2) (see note 2) |
Dempsey Corporation |
Data in respect of vapour pressure (5) (see note 3) Data in respect of hydrolysis rate as a function of pH (5) (see note 4) Data from an adsorption-desorption screening test (5) (see note 5) Data from an in vitro test for chromosomal aberrations in mammalian cells(5) (see note 6) |
Hexion Canada Inc. |
Data in respect of ready biodegradation |
Sherwin-Williams |
Data in respect of hydrolysis rate as a function of pH |
- Note 1
The number in brackets indicates the number of times that the information requirement in the second column was waived for the company. - Note 2
The number in brackets indicates the number of times that the information requirement in the second column was waived for the company. - Note 3
The number in brackets indicates the number of times that the information requirement in the second column was waived for the company. - Note 4
The number in brackets indicates the number of times that the information requirement in the second column was waived for the company. - Note 5
The number in brackets indicates the number of times that the information requirement in the second column was waived for the company. - Note 6
The number in brackets indicates the number of times that the information requirement in the second column was waived for the company.
EXPLANATORY NOTE
The decision to grant a waiver is made on a case-by-case basis by Environment Canada in consultation with Health Canada. On average, approximately 100 waivers are granted yearly for chemicals and polymers and living organisms for an average of 500 notifications received.
For more information, please see the waivers web page on the New Substances website.
[52-1-o]
DEPARTMENT OF THE ENVIRONMENT
DEPARTMENT OF HEALTH
CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999
Publication after screening assessment of nine substances in the Benzoates Group specified on the Domestic Substances List (paragraphs 68(b) and (c) or subsection 77(1) of the Canadian Environmental Protection Act, 1999)
Whereas the seven substances identified in the annex below are substances identified under subsection 73(1) of the Canadian Environmental Protection Act, 1999;
Whereas a summary of the draft screening assessment conducted on two substances pursuant to paragraphs 68(b) and (c) of the Act and on seven substances pursuant to section 74 of the Act is annexed hereby;
And whereas it is proposed to conclude that the substances do not meet any of the criteria set out in section 64 of the Act,
Notice therefore is hereby given that the Minister of the Environment and the Minister of Health (the ministers) propose to take no further action at this time under section 77 of the Act for the seven substances identified under subsection 73(1) of the Act.
Notice is also hereby given that the ministers propose to take no further action at this time for the two substances whose draft screening assessment was conducted pursuant to paragraphs 68(b) and (c) of the Act.
Public comment period
Any person may, within 60 days after publication of this notice, file with the Minister of the Environment written comments on the measure the ministers propose to take and on the scientific considerations on the basis of which the measure is proposed. More information regarding the scientific considerations may be obtained from the Canada.ca (Chemical Substances) website. All comments must cite the Canada Gazette, Part I, and the date of publication of this notice and be sent to the Executive Director, Program Development and Engagement Division, Department of the Environment, Gatineau, Quebec K1A 0H3, by fax to 819-938-5212, or by email to eccc.substances.eccc@canada.ca.
In accordance with section 313 of the Canadian Environmental Protection Act, 1999, any person who provides information in response to this notice may submit with the information a request that it be treated as confidential.
Jacqueline Gonçalves
Director General
Science and Risk Assessment Directorate
On behalf of the Minister of the Environment
David Morin
Director General
Safe Environments Directorate
On behalf of the Minister of Health
ANNEX
Summary of the draft screening assessment of nine substances in the Benzoates Group
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 9 of 10 substances referred to collectively under the Chemicals Management Plan as the Benzoates Group. These 9 substances were identified as priorities for assessment, as they met the categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. One of the 10 substances was subsequently determined to be of low concern for risk to ecological or human health and the decision for this substance is provided in a separate report. (see footnote 70) Accordingly, this screening assessment addresses the 9 substances listed in the table below.
| CAS RN (see note *) | Domestic Substances List name | Common name |
|---|---|---|
| 93-58-3 | Benzoic acid, methyl ester | Methyl benzoate |
| 93-89-0 (see note a) | Benzoic acid, ethyl ester | Ethyl benzoate |
| 120-50-3 (see note b) | Benzoic acid, 2-methylpropyl ester | Isobutyl benzoate |
| 120-55-8 | Ethanol, 2,2′-oxybis-, dibenzoate | Diethylene glycol dibenzoate |
| 136-60-7 | Benzoic acid, butyl ester | Butyl benzoate |
| 614-33-5 | 1,2,3-Propanetriol, tribenzoate | Tribenzoin |
| 8024-05-3(see note c) | Oils, tuberose | Tuberose oil |
| 27138-31-4 | Propanol, oxybis-, dibenzoate | Dipropylene glycol dibenzoate |
| 68052-23-3 | 1,3-Pentanediol, 2,2,4-trimethyl-, dibenzoate | Trimethylpentanediyl dibenzoate |
- Note *
The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society. - Note a
This substance was not identified under subsection 73(1) of CEPA but was included in this assessment, as it was considered a priority on the basis of other human health concerns. - Note b
This substance was not identified under subsection 73(1) of CEPA but was included in this assessment, as it was considered a priority on the basis of other human health concerns. - Note c
This substance is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
All nine substances in this assessment were included in a survey issued pursuant to section 71 of CEPA. Reported imported quantities ranged from 1 000 to 10 000 000 kg for five of the substances. Manufacturing and import activities were not reported for the four remaining substances.
Tribenzoin, tuberose oils, and methyl, ethyl, butyl, and isobutyl benzoates are used as food flavouring agents globally and are present in products available to consumers in Canada. These substances are also used as fragrance ingredients in household cleaning products and cosmetics. In addition to anthropogenic sources, methyl, ethyl, butyl, and isobutyl benzoates are naturally present in foods such as apples, bananas, sweet cherries, papayas, beer, cider, and cocoa.
Diethylene glycol dibenzoate, dipropylene glycol dibenzoate, and trimethylpentanediyl dibenzoate were identified in products including caulking, paint, and adhesives, as well as cosmetics and natural health products. Diethylene glycol dibenzoate and dipropylene glycol dibenzoate have also been identified as components in the manufacture of food packaging materials.
The ecological risks of the nine benzoates in this screening assessment were characterized using the ecological risk classification (ERC) of organic substances approach, which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles based principally on metrics regarding mode of toxic action, chemical reactivity, food web–derived internal toxicity thresholds, bioavailability, and chemical and biological activity are established. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. The ERC identified the nine benzoates in this screening assessment as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is a low risk of harm to organisms and the broader integrity of the environment from the nine benzoates addressed in this screening assessment. It is proposed to conclude that substances in the Benzoates Group do not meet the criteria under paragraph 64(a) or (b) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The characterization of the health effects in this assessment takes into consideration empirical evidence that benzoates readily hydrolyze into benzoic acid, which is then further metabolized into hippuric acid and subsequently excreted. Accordingly, the evaluation of the benzoate esters in this draft assessment focuses on health effects data for benzoic acid and benzyl derivatives considered to metabolize to benzoic acid. Taking into consideration the assessments of other jurisdictions, which have concluded that these substances and other similar substances show low toxicity, and given that the substances in this assessment metabolize to benzoic acid, the potential risk to human health is considered to be low.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that the substances in the Benzoates Group do not meet the criteria under paragraph 64(c) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Proposed conclusion
It is proposed to conclude that the nine substances in the Benzoates Group do not meet any of the criteria set out in section 64 of CEPA.
The draft screening assessment for these substances is available on the Canada.ca (Chemical Substances) website.
[52-1-o]
ENVIRONMENT AND CLIMATE CHANGE CANADA
SPECIES AT RISK ACT
Description of critical habitat for the Piping Plover, melodus subspecies (Charadrius melodus melodus) in Big Glace Bay Lake Bird Sanctuary and Black Pond Bird Sanctuary
This publication is an additional description of critical habitat for Piping Plover (Charadrius melodus melodus) and is independent from the two descriptions of critical habitat for that species published in Part I of the Canada Gazette on January 5, 2013, and June 25, 2016.
The Piping Plover melodus subspecies (Charadrius melodus melodus) is a small, stocky migratory shorebird protected under the Migratory Birds Convention Act, 1994 and listed on Schedule 1 of the Species at Risk Act. In Canada, the Piping Plover melodus subspecies nests on wide sand, gravel, or cobble beaches, barrier island sandspits, or peninsulas in marine coastal areas and is found along the coast of Newfoundland and Labrador, New Brunswick, Nova Scotia, Prince Edward Island and Quebec.
The Recovery Strategy for the Piping Plover (Charadrius melodus melodus) in Canada, available at http://www.sararegistry.gc.ca/species/speciesDetails_e.cfm?sid=687, identifies critical habitat for the species in a number of areas, including within federally protected areas.
Notice is hereby given that, pursuant to subsection 58(2) of the Species at Risk Act, subsection 58(1) of that Act applies, 90 days after the publication of this notice, to the critical habitat of the Piping Plover melodus subspecies — identified in the recovery strategy for that species that is included on the Species at Risk Public Registry — that is found within Big Glace Bay Lake Bird Sanctuary (also known as Glace Bay Bar) and Black Pond Bird Sanctuary described in the Migratory Bird Sanctuary Regulations made pursuant to the Migratory Birds Convention Act, 1994.
Interested parties are invited to contact Environment and Climate Change Canada by email at ec.protectionep-sarprotection.ec@canada.ca to request clarification regarding the location, biophysical attributes and protection of this species' critical habitat. However, some details may be withheld to protect the species and its critical habitat.
December 30, 2017
Mary Jane Roberts
Director
Species at Risk Act Management and Regulatory Affairs
Canadian Wildlife Service
[52-1-o]
ENVIRONMENT AND CLIMATE CHANGE CANADA
SPECIES AT RISK ACT
Description of Lewis's Woodpecker critical habitat in Vaseux-Bighorn National Wildlife Area and Vaseux Lake Bird Sanctuary
The Lewis's Woodpecker (Melanerpes lewis) is a migratory bird protected under the Migratory Birds Convention Act, 1994 and listed on Schedule 1 of the Species at Risk Act as threatened. In Canada, the Lewis's Woodpecker is typically found in open, mature ponderosa pine forests; riparian black cottonwood stands adjacent to open areas; and recently logged or burned coniferous forests with standing snags in the Okanagan, Similkameen, Thompson, and Boundary regions of British Columbia.
The Recovery Strategy for the Lewis's Woodpecker (Melanerpes lewis) in Canada, available at http://www.sararegistry.gc.ca/species/speciesDetails_e.cfm?sid=589, identifies critical habitat for the species in a number of areas, including within two federally protected areas.
Notice is hereby given that, pursuant to subsection 58(2) of the Species at Risk Act, subsection 58(1) of that Act applies, 90 days after the publication of this notice, to the critical habitat of the Lewis's Woodpecker — identified in the recovery strategy for that species that is included on the Species at Risk Public Registry — that is found within Vaseux-Bighorn National Wildlife Area, the boundaries of which are described in Schedule 1 of the Wildlife Area Regulations made pursuant to the Canada Wildlife Act, and Vaseux Lake Bird Sanctuary, described in the schedule of the Migratory Bird Sanctuary Regulations made pursuant to the Migratory Birds Convention Act, 1994.
Interested parties are invited to contact Environment and Climate Change Canada by email at ec.protectionep-sarprotection.ec@canada.ca to request clarification regarding the location, biophysical attributes and protection of this species' critical habitat. However, some details may be withheld to protect the species and its critical habitat.
December 30, 2017
Mary Jane Roberts
Director
Species at Risk Act Management and Regulatory Affairs
Canadian Wildlife Service
[52-1-o]
INNOVATION, SCIENCE AND ECONOMIC DEVELOPMENT CANADA
RADIOCOMMUNICATION ACT
Notice No. SLPB-010-17 — Extension to the comment period: Consultation on the Spectrum Outlook 2018 to 2022
Notice No. SLPB-006-17, Consultation on the Spectrum Outlook 2018 to 2022, was published on Innovation, Science and Economic Development Canada's (ISED) Spectrum Management and Telecommunications website on October 6, 2017. The deadline for submission of comments was indicated as January 9, 2018, and the deadline for submission of reply comments was indicated as February 8, 2018.
The purpose of the present notice is to advise all interested parties that based on the merits of several requests for additional time to respond, the deadline for submission of comments has been extended to February 16, 2018, and the deadline for reply comments has been extended to March 16, 2018. All comments received will be posted on ISED's Spectrum Management and Telecommunications website.
Obtaining copies
Copies of this notice and of documents referred to herein are available electronically on the Spectrum Management and Telecommunications website.
Official versions of notices can be viewed on the Canada Gazette website.
December 20, 2017
Chantal Davis
Acting Director
Spectrum Licensing Policy Branch
[52-1-o]
PRIVY COUNCIL OFFICE
Appointment opportunities
We know that our country is stronger — and our government more effective — when decision-makers reflect Canada's diversity. The Government of Canada will use an appointment process that is transparent and merit-based, strives for gender parity, and ensures that Indigenous peoples and minority groups are properly represented in positions of leadership. We will continue to search for Canadians who reflect the values that we all embrace: inclusion, honesty, fiscal prudence, and generosity of spirit. Together, we will build a government as diverse as Canada.
The Government of Canada is currently seeking applications from diverse and talented Canadians from across the country who are interested in the following positions.
Current opportunities
The following opportunities for appointments to Governor in Council positions are currently open for applications. Every opportunity is open for a minimum of two weeks from the date of posting on the Governor in Council Appointments website.
| Position | Organization | Closing date |
|---|---|---|
| President and Chief Executive Officer | Atomic Energy of Canada Limited | |
| Chief Executive Officer | Canadian Air Transport Security Authority | |
| Chief Executive Officer | Canadian Dairy Commission | |
| Members (appointment to roster) | International Trade and International Investment Dispute Settlement Bodies | |
| Parliamentary Librarian | Library of Parliament | |
| Chief Electoral Officer | Office of the Chief Electoral Officer | |
| Information Commissioner | Office of the Information Commissioner | |
| Commissioner | Royal Canadian Mounted Police | |
| Chairperson | Social Security Tribunal | January 9, 2018 |
Ongoing opportunities
| Position | Organization | Closing date |
|---|---|---|
| Full-time and Part-time Members | Immigration and Refugee Board | December 31, 2017 |
| Members | Veterans Review and Appeal Board | December 31, 2017 |
Upcoming opportunities
| Position | Organization |
|---|---|
| Chairperson | Civilian Review and Complaints Commission for the Royal Canadian Mounted Police |
| Sergeant-at-Arms | House of Commons |
| Commissioner | International Joint Commission |
[52-1-o]
TREASURY BOARD SECRETARIAT
MEMBERS OF PARLIAMENT RETIRING ALLOWANCES ACT
2018, 2019 and 2020 member contribution rates for the Members of Parliament pension plan
In accordance with subsection 2.7(10) of the Members of Parliament Retiring Allowances Act, the contribution rates, (see footnote 71) for calendar years 2018, 2019 and 2020, fixed under subsection 2.7(1) of the Act are as follows:
A. Contribution rates prior to reaching the 75% maximum pension accrual
| Calendar Year | Under Age 71 | Ages 71 and Above | Combined | |||
|---|---|---|---|---|---|---|
| Below YMPE (see footnote 72) | YMPE to MPE (see footnote 73) | Above MPE (see footnote 74) | Combined (see footnote 75) | |||
| 2018 | 11.13% | 14.22% | 0.00% | 11.24% | 0.00% | 10.69% |
| 2019 | 11.19% | 14.29% | 0.00% | 11.35% | 0.00% | 10.81% |
| 2020 | 11.30% | 14.43% | 0.00% | 11.50% | 0.00% | 10.94% |
| Calendar Year | Under Age 71 | Ages 71 and Above | Combined | ||
|---|---|---|---|---|---|
| Below MPE (see footnote 76) | Above MPE (see footnote 77) | Combined (see footnote 78) | |||
| 2018 | 6.31% | 19.41% | 8.17% | 19.41% | 8.72% |
| 2019 | 6.35% | 19.52% | 8.17% | 19.52% | 8.71% |
| 2020 | 6.40% | 19.70% | 8.20% | 19.70% | 8.76% |
| Calendar Years 2018, 2019 and 2020 | MPRA | MPRCA |
|---|---|---|
| Members Under Age 71 | 1.00% (salary up to MPE (see footnote 79)) | 1.00% (salary above the MPE (see footnote 80)) |
| Members 71 and Above | 0.00% | 1.00% |
Scott Brison
President of the Treasury Board
[52-1-o]